Immune Netw.
2011 Dec;11(6):416-419. 10.4110/in.2011.11.6.416.
Th17 Responses Are Not Induced in Dextran Sodium Sulfate Model of Acute Colitis
- Affiliations
-
- 1Department of Biomedical Laboratory Science, College of Health Sciences, Yonsei University at Wonju, Wonju 220-710, Korea. kjrhee@yonsei.ac.kr
- 2Department of Biomedical Laboratory Science, Daegu Health College, Daegu 702-722, Korea.
- KMID: 2150729
- DOI: http://doi.org/10.4110/in.2011.11.6.416
Abstract
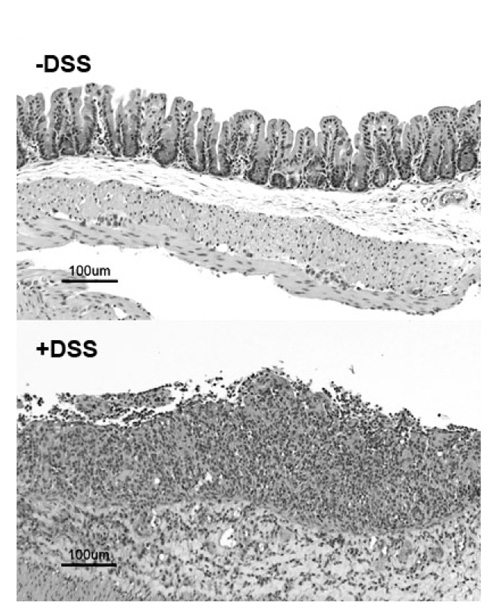
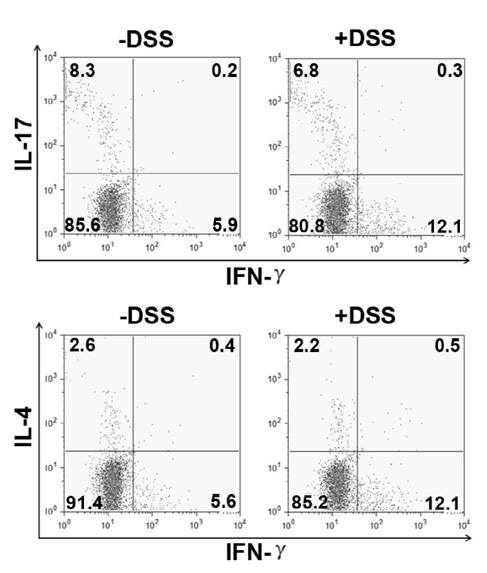
- Dextran sodium sulfate (DSS) is a widely used chemical model for inflammatory bowel disease (IBD). It is thought that imbalances in the T helper (Th) cell subsets contribute to IBD. Recent studies suggest that the acute DSS-colitis model is polarized toward a Th1/Th17 profile based on RT-PCR analysis of colonic tissues. In the current study we determined whether colonic Th cells from DSS-colitis mice were skewed toward the Th17 profile. Mice were treated with 5% DSS for 7 days and colonic T cells isolated and examined for production of IFN-gamma (Th1 cell), IL-4 (Th2 cell) and IL-17 (Th17 cell) by intracellular flow cytometry. We found that the percentage of colonic Th17 cells were similar to non-treated controls but the percentage of Th1 cells were elevated in DSS-colitis mice. These results suggest that in the acute DSS-colitis model the colonic Th cells exhibit a Th1 profile and not a Th17 profile.
MeSH Terms
Figure
Reference
-
1. Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007. 448:427–434.
Article2. Strober W, Fuss I, Mannon P. The fundamental basis of inflammatory bowel disease. J Clin Invest. 2007. 117:514–521.
Article3. Strober W. Why study animal models of IBD? Inflamm Bowel Dis. 2008. 14:Suppl 2. S129–S131.
Article4. Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990. 98:694–702.
Article5. Pizarro TT, Cominelli F. Cytokine therapy for Crohn's disease: advances in translational research. Annu Rev Med. 2007. 58:433–444.
Article6. Fuss IJ, Becker C, Yang Z, Groden C, Hornung RL, Heller F, Neurath MF, Strober W, Mannon PJ. Both IL-12p70 and IL-23 are synthesized during active Crohn's disease and are down-regulated by treatment with anti-IL-12 p40 monoclonal antibody. Inflamm Bowel Dis. 2006. 12:9–15.
Article7. Alex P, Zachos NC, Nguyen T, Gonzales L, Chen TE, Conklin LS, Centola M, Li X. Distinct cytokine patterns identified from multiplex profiles of murine DSS and TNBS-induced colitis. Inflamm Bowel Dis. 2009. 15:341–352.
Article8. Wu S, Rhee KJ, Albesiano E, Rabizadeh S, Wu X, Yen HR, Huso DL, Brancati FL, Wick E, McAllister F, Housseau F, Pardoll DM, Sears CL. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009. 15:1016–1022.
Article9. Brown JB, Cheresh P, Zhang Z, Ryu H, Managlia E, Barrett TA. P-selectin glycoprotein ligand-1 is needed for sequential recruitment of T-helper 1 (Th1) and local generation of Th17 T cells in dextran sodium sulfate (DSS) colitis. Inflamm Bowel Dis. 2011. [Epub ahead of print].
Article10. Sanada Y, Mizushima T, Kai Y, Nishimura J, Hagiya H, Kurata H, Mizuno H, Uejima E, Ito T. Therapeutic effects of novel sphingosine-1-phosphate receptor agonist W-061 in murine DSS colitis. PLoS One. 2011. 6:e23933.
Article11. Nuñez-Andrade N, Lamana A, Sancho D, Gisbert JP, Gonzalez-Amaro R, Sanchez-Madrid F, Urzainqui A. P-selectin glycoprotein ligand-1 modulates immune inflammatory responses in the enteric lamina propria. J Pathol. 2011. 224:212–221.
Article12. Ito R, Kita M, Shin-Ya M, Kishida T, Urano A, Takada R, Sakagami J, Imanishi J, Iwakura Y, Okanoue T, Yoshikawa T, Kataoka K, Mazda O. Involvement of IL-17A in the pathogenesis of DSS-induced colitis in mice. Biochem Biophys Res Commun. 2008. 377:12–16.
Article13. Egger B, Bajaj-Elliott M, MacDonald TT, Inglin R, Eysselein VE, Büchler MW. Characterisation of acute murine dextran sodium sulphate colitis: cytokine profile and dose dependency. Digestion. 2000. 62:240–248.
Article14. Nam JS, Terabe M, Kang MJ, Chae H, Voong N, Yang YA, Laurence A, Michalowska A, Mamura M, Lonning S, Berzofsky JA, Wakefield LM. Transforming growth factor beta subverts the immune system into directly promoting tumor growth through interleukin-17. Cancer Res. 2008. 68:3915–3923.
Article15. Pichavant M, Goya S, Meyer EH, Johnston RA, Kim HY, Matangkasombut P, Zhu M, Iwakura Y, Savage PB, DeKruyff RH, Shore SA, Umetsu DT. Ozone exposure in a mouse model induces airway hyperreactivity that requires the presence of natural killer T cells and IL-17. J Exp Med. 2008. 205:385–393.
Article16. Rachitskaya AV, Hansen AM, Horai R, Li Z, Villasmil R, Luger D, Nussenblatt RB, Caspi RR. Cutting edge: NKT cells constitutively express IL-23 receptor and RORgammat and rapidly produce IL-17 upon receptor ligation in an IL-6-independent fashion. J Immunol. 2008. 180:5167–5171.
Article17. Ferretti S, Bonneau O, Dubois GR, Jones CE, Trifilieff A. IL-17, produced by lymphocytes and neutrophils, is necessary for lipopolysaccharide-induced airway neutrophilia: IL-15 as a possible trigger. J Immunol. 2003. 170:2106–2112.
Article18. Takahashi N, Vanlaere I, de Rycke R, Cauwels A, Joosten LA, Lubberts E, van den Berg WB, Libert C. IL-17 produced by Paneth cells drives TNF-induced shock. J Exp Med. 2008. 205:1755–1761.
Article19. Duan J, Chung H, Troy E, Kasper DL. Microbial colonization drives expansion of IL-1 receptor 1-expressing and IL-17-producing gamma/delta T cells. Cell Host Microbe. 2010. 7:140–150.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Decursinol Angelate Ameliorates Dextran Sodium Sulfate-Induced Colitis by Modulating Type 17 Helper T Cell Responses
- Histological Study of Experimental Colitis Induced by Dextran Sulfate Sodium
- Fine Structure of Goblet Cell Regeneration on Experimental Colitis Induced by Dextran Sulfate Sodium
- Anti-inflammatory effects of mulberry twig extracts on dextran sulfate sodium-induced colitis mouse model
- Benzoxazole Derivative B-98 Ameliorates Dextran Sulfate Sodium-induced Acute Murine Colitis and the Change of T Cell Profiles in Acute Murine Colitis Model