Immune Netw.
2010 Apr;10(2):35-45. 10.4110/in.2010.10.2.35.
Expression of a Functional zipFv Antibody Fragment and Its Fusions with Alkaline Phosphatase in the Cytoplasm of an Escherichia coli
- Affiliations
-
- 1Division of Molecular & Medical Biotechnology, College of Biomedical Science, Kangwon National University, Chuncheon 200-701, Korea.
- 2IG Therapy Co., Kangwon National University, Chuncheon 200-701, Korea. chash@kangwon.ac.kr
- 3Department of System Immunology, College of Biomedical Science, Kangwon National University, Chuncheon 200-701, Korea.
- KMID: 2150658
- DOI: http://doi.org/10.4110/in.2010.10.2.35
Abstract
-
BACKGROUND: Expression of recombinant antibodies and their derivatives fused with other functional molecules such as alkaline phosphatase in Escherichia coli is important in the development of molecular diagnostic reagents for biomedical research.
METHODS
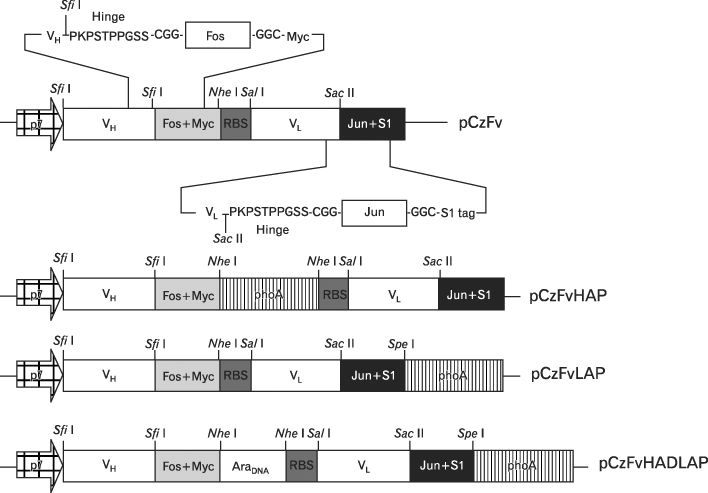
We investigated the possibility of applying a well-known Fos-Jun zipper to dimerize V(H) and V(L) fragments originated from the Fab clone (SP 112) that recognizes pyruvate dehydrogenase complex-E2 (PDC-E2), and demonstrated that the functional zipFv-112 and its alkaline phosphatase fusion molecules (zipFv-AP) can be produced in the cytoplasm of Origami(DE3) trxB gor mutant E. coli strain.
RESULTS
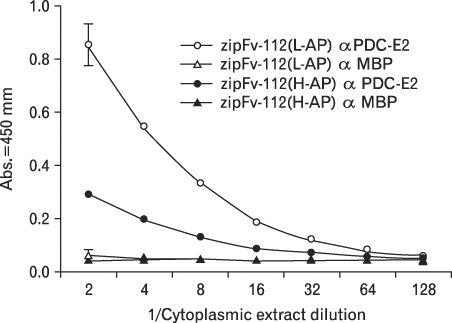
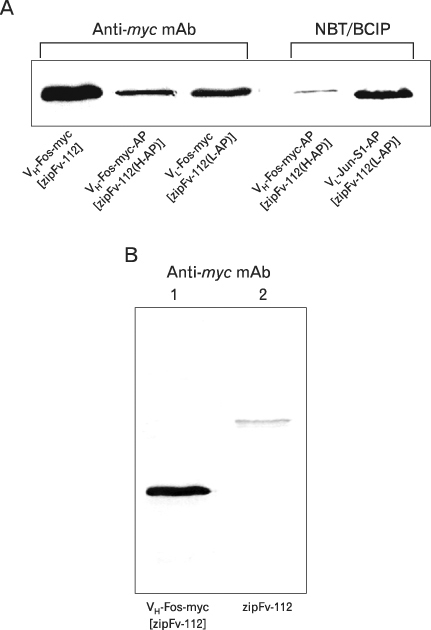
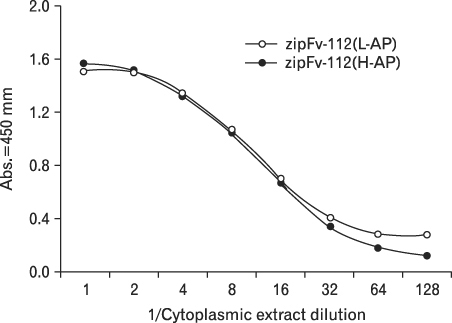
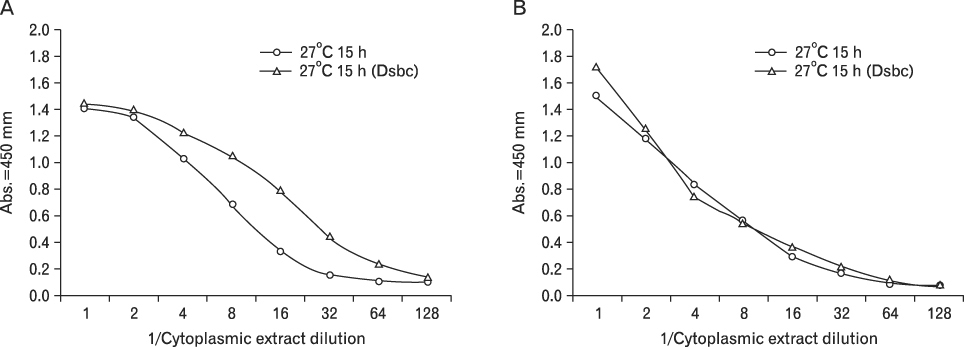
The zipFv-AP fusion molecules exhibited higher antigen-binding signals than the zipFv up to a 10-fold under the same experimental conditions. However, conformation of the zipFv-AP seemed to be influenced by the location of an AP domain at the C-terminus of V(H) or V(L) domain [zipFv-112(H-AP) or zipFv-112(L-AP)], and inclusion of an AraC DNA binding domain at the C-terminus of V(H) of the zipFv-112(L-AP), termed zipFv-112(H-AD/L-AP), was also beneficial. Cytoplasmic co-expression of disulfide-binding isomerase C (DsbC) helped proper folding of the zipFv-112(H-AD/L-AP) but not significantly.
CONCLUSION
We believe that our zipFv constructs may serve as an excellent antibody format bi-functional antibody fragments that can be produced stably in the cytoplasm of E. coli.
Keyword
MeSH Terms
-
Alkaline Phosphatase
Antibodies
Clone Cells
Cytoplasm
DNA
Escherichia
Escherichia coli
Immunoglobulin Fragments
Indicators and Reagents
Leucine Zippers
Oxidoreductases
Pathology, Molecular
Pyruvic Acid
Sprains and Strains
Alkaline Phosphatase
Antibodies
DNA
Immunoglobulin Fragments
Indicators and Reagents
Oxidoreductases
Pyruvic Acid
Figure
Reference
-
1. Morino K, Katsumi H, Akahori Y, Iba Y, Shinohara M, Ukai Y, Kohara Y, Kurosawa Y. Antibody fusions with fluorescent proteins: a versatile reagent for profiling protein expression. J Immunol Methods. 2001. 257:175–184.
Article2. Pack P, Plückthun A. Miniantibodies use of amphipathic helices to produce functional, flexibly linked dimeric Fv fragments with high avidity in Escherichia coli. Biochemistry. 1992. 31:1579–1584.
Article3. Horne C, Klein M, Polidoulis I, Dorrington KJ. Noncovalent association of heavy and light chains of human immunoglobulins. III. Specific interactions between VH and VL. J Immunol. 1982. 129:660–664.4. Jäger M, Plückthun A. Domain interactions in antibody Fv and scFv fragments: effects on unfolding kinetics and equilibria. FEBS Lett. 1999. 462:307–312.
Article5. Jäger M, Plückthun A. Folding and assembly of an antibody Fv fragment, a heterodimer stabilized by antigen. J Mol Biol. 1999. 285:2005–2019.
Article6. Huston JS, Levinson D, Mudgett-Hunter M, Tai MS, Novotný J, Margolies MN, Ridge RJ, Bruccoleri RE, Haber E, Crea R, et al. Protein engineering of antibody binding sites: recovery of specific activity in an anti-digoxin single-chain Fv analogue produced in Escherichia coli. Proc Natl Acad Sci U S A. 1988. 85:5879–5883.
Article7. Bird RE, Hardman KD, Jacobson JW, Johnson S, Kaufman BM, Lee SM, Lee T, Pope SH, Riordan GS, Whitlow M. Single-chain antigen-binding proteins. Science. 1988. 242:423–426.
Article8. Chaudhary VK, Queen C, Junghans RP, Waldmann TA, FitzGerald DJ, Pastan I. A recombinant immunotoxin consisting of two antibody variable domains fused to Pseudomonas exotoxin. Nature. 1989. 339:394–397.
Article9. Glockshuber R, Malia M, Pfitzinger I, Pluckthun A. A comparison of strategies to stabilize immunoglobulin Fv-fragments. Biochemistry. 1990. 29:1362–1367.
Article10. Bird RE, Walker BW. Single chain antibody variable regions. Trends Biotechnol. 1991. 9:132–137.
Article11. Reiter Y, Brinkmann U, Jung SH, Lee B, Kasprzyk PG, King CR, Pastan I. Improved binding and antitumor activity of a recombinant anti-erbB2 immunotoxin by disulfide stabilization of the Fv fragment. J Biol Chem. 1994. 269:18327–18331.
Article12. Jung SH, Pastan I, Lee B. Design of interchain disulfide bonds in the framework region of the Fv fragment of the monoclonal antibody B3. Proteins. 1994. 19:35–47.
Article13. Reiter Y, Brinkmann U, Lee B, Pastan I. Engineering antibody Fv fragments for cancer detection and therapy: disulfide-stabilized Fv fragments. Nat Biotechnol. 1996. 14:1239–1245.
Article14. Reiter Y, Brinkmann U, Kreitman RJ, Jung SH, Lee B, Pastan I. Stabilization of the Fv fragments in recombinant immunotoxins by disulfide bonds engineered into conserved framework regions. Biochemistry. 1994. 33:5451–5459.
Article15. Reiter Y, Brinkmann U, Webber KO, Jung SH, Lee B, Pastan I. Engineering interchain disulfide bonds into conserved framework regions of Fv fragments improved biochemical characteristics of recombinant immunotoxins containing disulfide-stabilized Fv. Protein Eng. 1994. 7:697–704.
Article16. Skerra A, Plückthun A. Assembly of a functional immunoglobulin Fv fragment in Escherichia coli. Science. 1988. 240:1038–1041.
Article17. Krebber A, Burmester J, Plückthun A. Inclusion of an up-stream transcriptional terminator in phage display vectors abolishes background expression of toxic fusions with coat protein g3p. Gene. 1996. 178:71–74.
Article18. Clark MA, Hammond FR, Papaioannou A, Hawkins NJ, Ward RL. Regulation and expression of human Fabs under the control of the Escherichia coli arabinose promoter, PBAD. Immunotechnol. 1997. 3:217–226.
Article19. Knappik A, Plückthun A. Engineered turns of a recombinant antibody improve its in vivo folding. Protein Eng. 1995. 8:81–89.20. Deramn AI, Prinz WA, Belin D, Beckwith J. Mutations that allow disulfide bond formation in the cytoplasm of Escherichia coli. Science. 1993. 262:1744–1747.
Article21. He M, Hamon M, Liu H, Kang A, Taussig MJ. Functional expression of a single-chain anti-progesterone antibody fragment in the cytoplasm of a mutant Escherichia coli. Nucleic Acids Res. 1995. 23:4009–4010.
Article22. Jurado P, Ritz D, Beckwith J, deLorenzo V, Fernandez LA. Production of functional single-chain Fv antibodies in the cytoplasm of Escherichia coli. J Mol Biol. 2002. 320:1–10.
Article23. Levy R, Weiss R, Chen G, Iverson BL, Georgiou G. Production of correctly folded Fab antibody fragment in the cytoplasm of Escherichia coli trxB gor mutants via the coexpression of molecular chaperones. Protein Expr Purif. 2001. 23:338–347.
Article24. Venturi M, Seifert C, Hunte C. High level production of functional antibody Fab fragments in an oxidizing bacterial cytoplasm. J Mol Biol. 2002. 315:1–8.
Article25. Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning. 1989. 2nd ed. NY, USA: Cold Spring Harbor Laboratory Press Cold Spring Harbor.26. Cha S, Leung PS, Coppel RL, VandeWater J, Ansari AA, Gershwin ME. Heterogeneity of combinatorial human auto-antibodies against PDC-E2 and biliary epithelial cells in patients with primary biliary cirrhosis. Hepatology. 1994. 20:574–583.
Article27. McCafferty J, Johnson KS. McCafferty J, Johnson KS, editors. Construction and screening of antibody display libraries. Phage Display of Peptides and Proteins. A Laboratory Manual. 1996. San Diego, CA, USA: Academic Press Inc.
Article28. Brunelle A, Schleif R. Determining residue-base interactions between AraC protein and araI DNA. J Mol Biol. 1989. 209:607–622.
Article29. Menon KP, Lee NL. Activation of ara operons by a truncated AraC protein does not require inducer. Proc Natl Acad Sci U S A. 1990. 87:3708–3712.
Article30. Cha S, Leung PS, Gershwin ME, Fletcher MP, Ansari AA, Coppel RL. Combinatorial autoantibodies to dihydrolipoamide acetyltransferase, the major autoantigen of primary biliary cirrhosis. Proc Natl Acad Sci U S A. 1993. 90:2527–2531.
Article31. O'shea EK, Rutkowski R, Stafford WE 3rd, Kim PS. Preferential heterodimer formation by isolated leucine zipper from fos and jun. Science. 1989. 245:646–648.32. Griep RA, vanTwisk C, Kerschbaumer RJ, Harper K, Torrance L, Himmler G, vanderWolf JM, Schots A. pSKAP/S: an expression vector for the production of single-chain Fv alkaline phosphatase fusion proteins. Protein Expr Purif. 1999. 16:63–69.
Article33. Hayhurst A. Improved expression characteristics of single-chain Fv fragments when fused downstream of the Escherichia coli maltose-binding protein or upstream of a single immunoglobulin-constant domain. Protein Expr Purif. 2000. 18:1–10.
Article34. Bustos SA, Schleif RF. Functional domains of the AraC protein. Proc Natl Acad Sci U S A. 1993. 90:5638–5642.
Article35. Strachan G, Williams S, Moyle SP, Harris WJ, Porter AJ. Reduced toxicity of expression, in Escherichia coli, of anti pollutant antibody fragments and their use as sensitive diagnostic molecules. J Appl Microbiol. 1999. 87:410–417.
Article36. Bessette PH, Aslund F, Beckwith J, Georgiou G. Efficient folding of proteins with multiple disulfide bonds in the Escherichia coli cytoplasm. Proc Natl Acad Sci U S A. 1999. 96:13703–13708.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of human CTL?4 extracellular domain in escherichia coli
- Escherichia coli O157: H7 Infection
- Identification and characterization of the fimbrial adhesin and gene product that regulates the expression of fimbriae in escherichia coli
- Leukocyte Alkaline Phosphatase Activity in Children with leukocytosis
- Characterization of the Gene for the Hemin-Binding Protein from Porphyromonas Gingivalis