Cancer Res Treat.
2015 Jul;47(3):406-415. 10.4143/crt.2014.073.
A Randomized Phase II Trial of Capecitabine Plus Vinorelbine Followed by Docetaxel Versus Adriamycin Plus Cyclophosphamide Followed by Docetaxel as Neoadjuvant Chemotherapy for Breast Cancer
- Affiliations
-
- 1Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. drjiny@amc.seoul.kr
- 2Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 3Department of Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 4Department of Radiation Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 5Department of Radiology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 2148489
- DOI: http://doi.org/10.4143/crt.2014.073
Abstract
- PURPOSE
Given the promising activity of capecitabine and vinorelbine in metastatic breast cancer, this randomized phase II trial evaluated the efficacy and safety of this combination as neoadjuvant chemotherapy in breast cancer.
MATERIALS AND METHODS
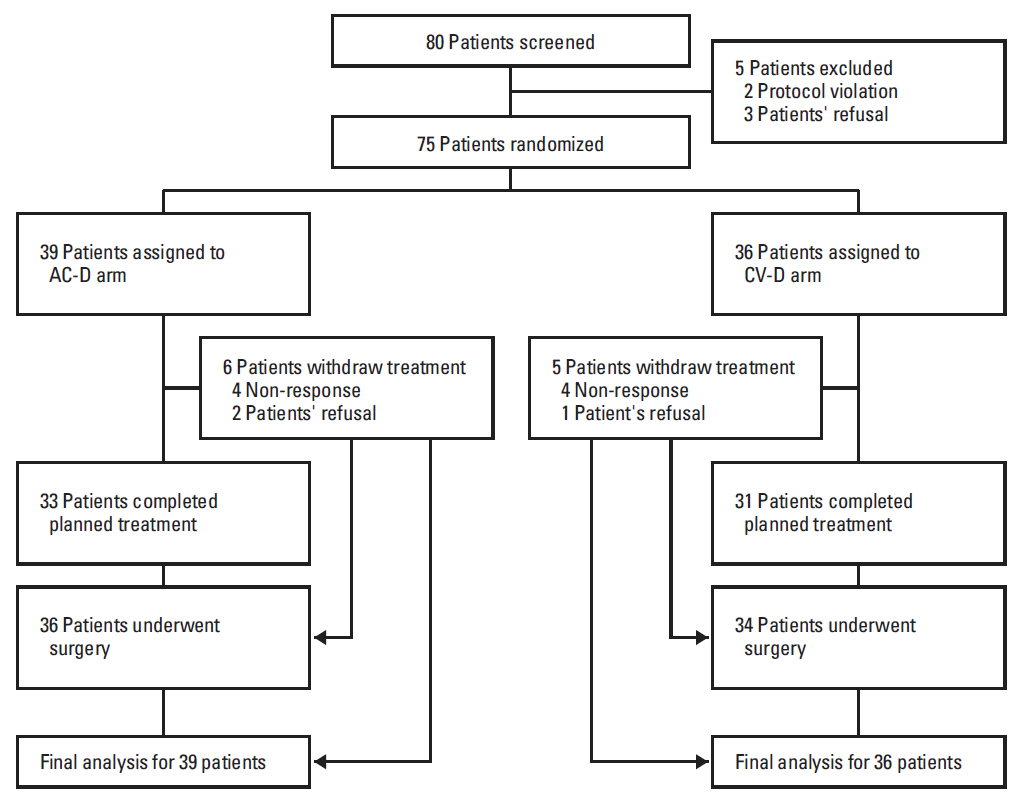
Patients with operable breast cancer (n=75) were randomly assigned to receive either four cycles of adriamycin 60 mg/m2 plus cyclophosphamide 600 mg/m2 every 3 weeks followed by four cycles of docetaxel 75 mg/m2 every 3 weeks (AC-D) or four cycles of capecitabine 2,000 mg/m2 (day 1-14) plus vinorelbine 25 mg/m2 (days 1 and 8) every 3 weeks followed by four cycles of docetaxel 75 mg/m2 (CV-D). The primary endpoint was pathologic complete response (pCR) in the primary breast (ypT0/is).
RESULTS
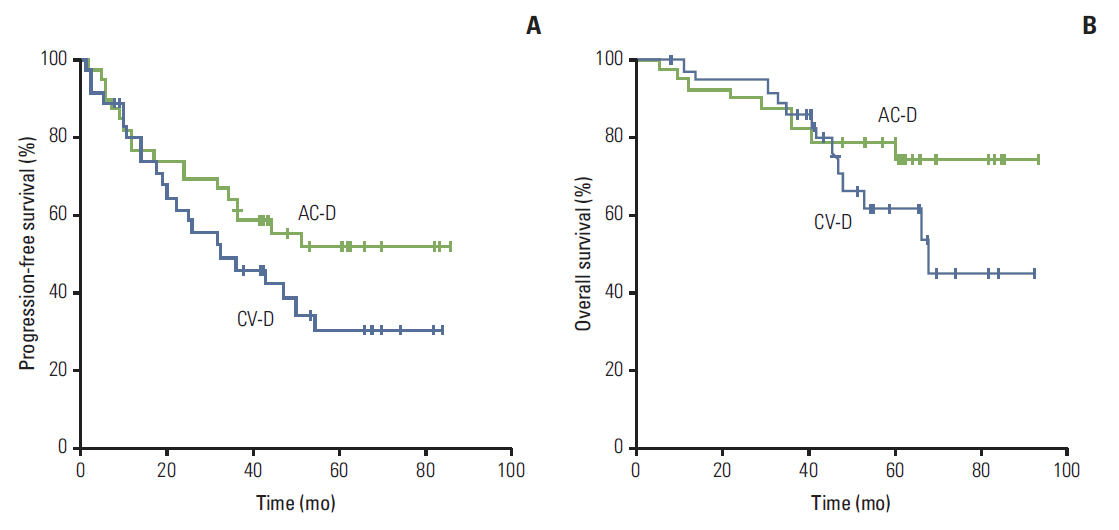
Most patients (84%) had locally advanced (n=41) or inflammatory breast cancer (n=22). pCR rates in the primary breast were 15% (95% confidence interval [CI], 7% to 30%) and 11% (95% CI, 4% to 26%) in the AC-D and CV-D groups, respectively. The overall response rates and 5-year progression-free survival rates in the AC-D and CV-D groups were 62% and 64%, and 51.3% (95% CI, 34.6% to 68.0%) and 30.2% (95% CI, 13.3% to 47.1%), respectively. Although both regimens were well tolerated, CV-D showed less frequent grade 3-4 neutropenia and vomiting than AC-D, whereas manageable diarrhea and hand-foot syndrome were more common in the CV-D group.
CONCLUSION
CV-D is a feasible and active non-anthracycline-based neoadjuvant chemotherapy regimen for breast cancer.
MeSH Terms
Figure
Reference
-
References
1. Schwartz GF, Birchansky CA, Komarnicky LT, Mansfield CM, Cantor RI, Biermann WA, et al. Induction chemotherapy followed by breast conservation for locally advanced carcinoma of the breast. Cancer. 1994; 73:362–9.
Article2. Mauriac L, Durand M, Avril A, Dilhuydy JM. Effects of primary chemotherapy in conservative treatment of breast cancer patients with operable tumors larger than 3 cm: results of a randomized trial in a single centre. Ann Oncol. 1991; 2:347–54.3. van der Hage JA, van de Velde CJ, Julien JP, Tubiana-Hulin M, Vandervelden C, Duchateau L. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol. 2001; 19:4224–37.
Article4. Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008; 26:778–85.
Article5. Martin M, Pienkowski T, Mackey J, Pawlicki M, Guastalla JP, Weaver C, et al. Adjuvant docetaxel for node-positive breast cancer. N Engl J Med. 2005; 352:2302–13.
Article6. Mackey JR, Martin M, Pienkowski T, Rolski J, Guastalla JP, Sami A, et al. Adjuvant docetaxel, doxorubicin, and cyclophosphamide in node-positive breast cancer: 10-year follow-up of the phase 3 randomised BCIRG 001 trial. Lancet Oncol. 2013; 14:72–80.
Article7. Bear HD, Anderson S, Smith RE, Geyer CE Jr, Mamounas EP, Fisher B, et al. Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer: National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2006; 24:2019–27.
Article8. Henderson IC. Can we abandon anthracyclines for early breast cancer patients? Oncology (Williston Park). 2011; 25:115–27.9. Giordano SH, Lin YL, Kuo YF, Hortobagyi GN, Goodwin JS. Decline in the use of anthracyclines for breast cancer. J Clin Oncol. 2012; 30:2232–9.
Article10. Wildiers H, Neven P, Christiaens MR, Squifflet P, Amant F, Weltens C, et al. Neoadjuvant capecitabine and docetaxel (plus trastuzumab): an effective non-anthracycline-based chemotherapy regimen for patients with locally advanced breast cancer. Ann Oncol. 2011; 22:588–94.
Article11. Lee KS, Ro J, Nam BH, Lee ES, Kwon Y, Kwon HS, et al. A randomized phase-III trial of docetaxel/capecitabine versus doxorubicin/cyclophosphamide as primary chemotherapy for patients with stage II/III breast cancer. Breast Cancer Res Treat. 2008; 109:481–9.
Article12. Jones SE, Savin MA, Holmes FA, O'Shaughnessy JA, Blum JL, Vukelja S, et al. Phase III trial comparing doxorubicin plus cyclophosphamide with docetaxel plus cyclophosphamide as adjuvant therapy for operable breast cancer. J Clin Oncol. 2006; 24:5381–7.
Article13. Chan A, Verrill M. Capecitabine and vinorelbine in metastatic breast cancer. Eur J Cancer. 2009; 45:2253–65.
Article14. Pajk B, Cufer T, Canney P, Ellis P, Cameron D, Blot E, et al. Anti-tumor activity of capecitabine and vinorelbine in patients with anthracycline- and taxane-pretreated metastatic breast cancer: findings from the EORTC 10001 randomized phase II trial. Breast. 2008; 17:180–5.
Article15. Sawada N, Fujimoto-Ouchi K, Ishikawa T, Tanaka Y, Ishituka H. Antitumor activity of combination therapy with capecitabine plus vinorelbine, and capecitabine plus gemcitabine in human tumor xenograft models. Proc Am Assoc Cancer Res. 2002; 43:1088a. (Abstr 5388).16. Ahn JH, Kim SB, Kim TW, Ahn SH, Kim SM, Park JM, et al. Capecitabine and vinorelbine in patients with metastatic breast cancer previously treated with anthracycline and taxane. J Korean Med Sci. 2004; 19:547–53.
Article17. Welt A, von Minckwitz G, Oberhoff C, Borquez D, Schleucher R, Loibl S, et al. Phase I/II study of capecitabine and vinorelbine in pretreated patients with metastatic breast cancer. Ann Oncol. 2005; 16:64–9.
Article18. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000; 92:205–16.19. Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989; 10:1–10.
Article20. von Minckwitz G, Kummel S, Vogel P, Hanusch C, Eidtmann H, Hilfrich J, et al. Neoadjuvant vinorelbine-capecitabine versus docetaxel-doxorubicin-cyclophosphamide in early nonresponsive breast cancer: phase III randomized GeparTrio trial. J Natl Cancer Inst. 2008; 100:542–51.
Article21. Yoo C, Ahn JH, Jung KH, Kim SB, Kim HH, Shin HJ, et al. Impact of immunohistochemistry-based molecular subtype on chemosensitivity and survival in patients with breast cancer following neoadjuvant chemotherapy. J Breast Cancer. 2012; 15:203–10.
Article22. Andre F, Mazouni C, Liedtke C, Kau SW, Frye D, Green M, et al. HER2 expression and efficacy of preoperative paclitaxel/FAC chemotherapy in breast cancer. Breast Cancer Res Treat. 2008. 108:183–90.
Article23. Kuerer HM, Newman LA, Smith TL, Ames FC, Hunt KK, Dhingra K, et al. Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol. 1999; 17:460–9.
Article24. Ring AE, Smith IE, Ashley S, Fulford LG, Lakhani SR. Oestrogen receptor status, pathological complete response and prognosis in patients receiving neoadjuvant chemotherapy for early breast cancer. Br J Cancer. 2004; 91:2012–7.
Article25. von Minckwitz G, Blohmer JU, Costa SD, Denkert C, Eidtmann H, Eiermann W, et al. Response-guided neoadjuvant chemotherapy for breast cancer. J Clin Oncol. 2013; 31:3623–30.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neoadjuvant Chemotherapy with Docetaxel and Adriamycin in Breast Cancer; Clincopathologic Factors Influencing to Response Rate
- Short Term Effect of Neoadjuvant Therapy with Docetaxel and Adriamycin in Advanced Breast Cancer
- A Multicenter Randomized Phase II Study of Docetaxel vs. Docetaxel Plus Cisplatin vs. Docetaxel Plus S-1 as Second-Line Chemotherapy in Metastatic Gastric Cancer Patients Who Had Progressed after Cisplatin Plus Either S-1 or Capecitabine
- A New Option for Advanced Gastric Cancer: Docetaxel and Novel Oral Fluoropyrimidine Combination Chemotherapy
- Incidence of Febrile Neutropenia in Korean Female Breast Cancer Patients Receiving Preoperative or Postoperative Doxorubicin/Cyclophosphamide Followed by Docetaxel Chemotherapy