Electrolyte Blood Press.
2008 Dec;6(2):77-85. 10.5049/EBP.2008.6.2.77.
Kidney and Phosphate Metabolism
- Affiliations
-
- 1Depatment of internal Medicine, Konyang University College of Medicine, Daejeon, Korea. cnw7799@hanmail.net
- KMID: 2134809
- DOI: http://doi.org/10.5049/EBP.2008.6.2.77
Abstract
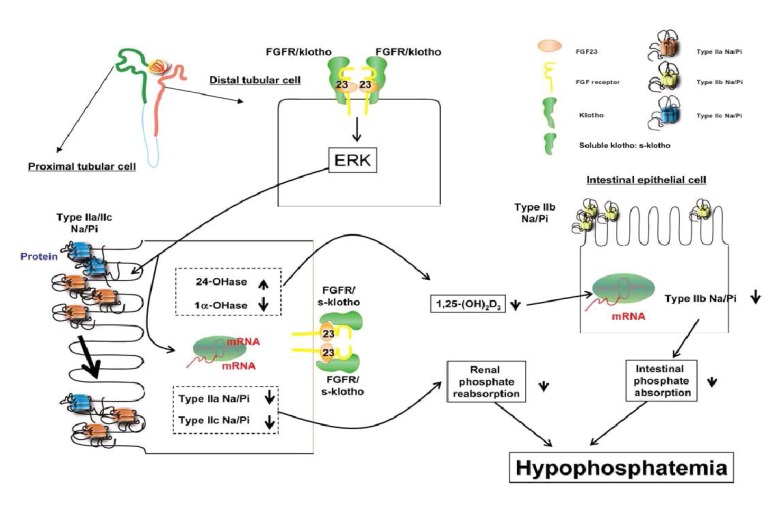
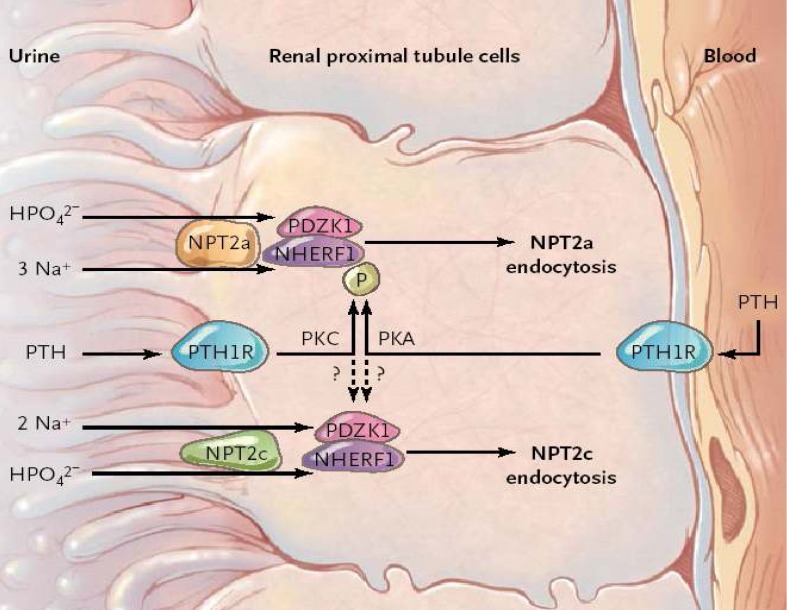
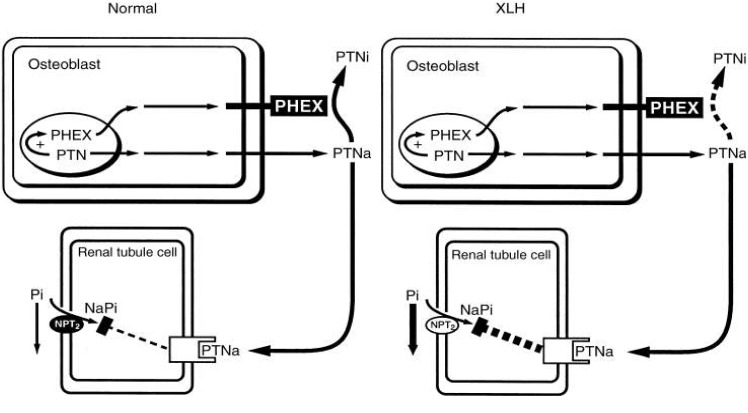
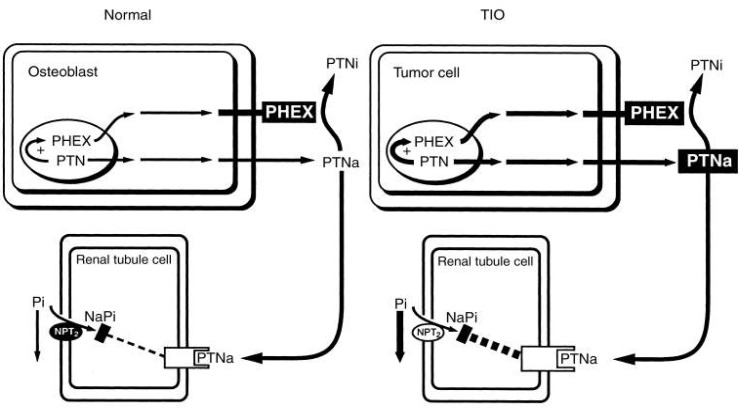
- The serum phosphorus level is maintained through a complex interplay between intestinal absorption, exchange intracellular and bone storage pools, and renal tubular reabsorption. The kidney plays a major role in regulation of phosphorus homeostasis by renal tubular reabsorption. Type IIa and type IIc Na+/Pi transporters are important renal Na+-dependent inorganic phosphate (Pi) transporters, which are expressed in the brush border membrane of proximal tubular cells. Both are regulated by dietary Pi intake, vitamin D, fibroblast growth factor 23 (FGF23) and parathyroid hormone. The expression of type IIa Na+/Pi transporter result from hypophosphatemia quickly. However, type IIc appears to act more slowly. Physiological and pathophysiological alteration in renal Pi reabsorption are related to altered brush-border membrane expression/content of the type II Na+/Pi cotransporter. Many studies of genetic and acquired renal phosphate wasting disorders have led to the identification of novel genes. Two novel Pi regulating genes, PHEX and FGF23, play a role in the pathophysiology of genetic and acquired renal phosphate wasting disorders and studies are underway to define their mechanism on renal Pi regulation. In recent studies, sodium-hydrogen exchanger regulatory factor 1 (NHERF1) is reported as another new regulator for Pi reabsorption mechanism.
MeSH Terms
-
Fibroblast Growth Factors
Homeostasis
Hypophosphatemia
Intestinal Absorption
Kidney
Membranes
Microvilli
Parathyroid Hormone
Phosphoproteins
Phosphorus
Sodium-Hydrogen Antiporter
Sodium-Phosphate Cotransporter Proteins
Vitamin D
Fibroblast Growth Factors
Parathyroid Hormone
Phosphoproteins
Phosphorus
Sodium-Hydrogen Antiporter
Sodium-Phosphate Cotransporter Proteins
Vitamin D
Figure
Cited by 1 articles
-
Vitamin D, and Kidney Disease
Hyung Soo Kim, Wookyung Chung, Sejoong Kim
Electrolyte Blood Press. 2011;9(1):1-6. doi: 10.5049/EBP.2011.9.1.1.
Reference
-
1. Berndt TJ, Knox FG. Seldin DW, Giebisch GH, editors. Renal regulation of phosphate excretion. The Kidney: Physiology and Pathophysiology. 1992. 2nd ed. Raven;p. 2511–2532.2. Dennis VW. Windhager EE, editor. Phosphate homeostasis. Renal physiology. 1992. New York American Physiological Society by Oxford University Press;p. 1785–1815.
Article3. Miller WL, Portale AA. Genetic causes of rickets. Curr Opin Pediatr. 1999; 11:333–339. PMID: 10439207.
Article4. Demay MB, Sabbagh Y, Carpenter TO. Calcium and vitamin D: what is known about the effects on growing bone. Pediatrics. 2007; 119(Suppl 2):S141–S144. PMID: 17332234.
Article5. Murer H, Hernando N, Forster I, Biber J. Proximal tubular phosphate reabsorption: molecular mechanisms. Physiol Rev. 2000; 80:1373–1409. PMID: 11015617.
Article6. Kempson SA. Peptide hormone action on renal phosphate handling. Kidney Int. 1996; 49:1005–1009. PMID: 8691715.
Article7. Soumounou Y, Gauthier C, Tenenhouse HS. Murine and human type I Na-phosphate cotransporter genes: structure and promoter activity. Am J Physiol Renal Physiol. 2001; 281:F1082–F1091. PMID: 11704559.8. Broer S, Schuster A, Wagner CA, Broer A, Forster I, Biber J, et al. Chloride conductance and Pi transport are separate functions induced by the expression of NaPi-1 in Xenopus oocytes. J Membr Biol. 1998; 164:71–77. PMID: 9636245.9. Zhao N, Tenenhouse HS. Npt2 gene disruption confers resistance to the inhibitory action of parathyroid hormone on renal sodium-phosphate cotransport. Endocrinology. 2000; 141:2159–2165. PMID: 10830304.
Article10. Murer H, Forster I, Biber J. The sodium phosphate cotransporter family SLC34. Pflugers Arch. 2004; 447:763–767. PMID: 12750889.
Article11. Tenenhouse HS. Phosphate transport: molecular basis, regulation and pathophysiology. J Steroid Biochem Mol Biol. 2007; 103:572–577. PMID: 17270430.
Article12. Beck L, Karaplis AC, Amizuka N, Hewson AS, Ozawa H, Tenenhouse HS. Targeted inactivation of Npt2 in mice leads to severe renal phosphate wasting, hypercalciuria, and skeletal abnormalities. Proc Natl Acad Sci U S A. 1998; 95:5372–5377. PMID: 9560283.
Article13. Hilfiker H, Hattenhauer O, Traebert M, Forster I, Murer H, Biber J. Characterization of a murine type II sodium-phosphate cotransporter expressed in mammalian small intestine. Proc Natl Acad Sci U S A. 1998; 95:14564–14569. PMID: 9826740.
Article14. Lotscher M, Scarpetta Y, Levi M, Halaihel N, Wang H, Zajicek HK, et al. Rapid downregulation of rat renal Na/P(i) cotransporter in response to parathyroid hormone involves microtubule rearrangement. J Clin Invest. 1999; 104:483–494. PMID: 10449440.15. Lorenz-Depiereux B, Benet-Pages A, Eckstein G, Tenenbaum-Rakover Y, Wagenstaller J, Tiosano D, et al. Hereditary hypophosphatemic rickets with hypercalciuria is caused by mutations in the sodium-phosphate cotransporter gene SLC34A3. Am J Hum Genet. 2006; 78:193–201. PMID: 16358215.
Article16. Bergwitz C, Roslin NM, Tieder M, Loredo-Osti JC, Bastepe M, Abu-Zahra H, et al. SLC34A3 mutations in patients with hereditary hypophosphatemic rickets with hypercalciuria predict a key role for the sodium-phosphate cotransporter NaPi-IIc in maintaining phosphate homeostasis. Am J Hum Genet. 2006; 78:179–192. PMID: 16358214.
Article17. Ichikawa S, Sorenson AH, Imel EA, Friedman NE, Gertner JM, Econs MJ. Intronic deletions in the SLC34A3 gene cause hereditary hypophosphatemic rickets with hypercalciuria. J Clin Endocrinol Metab. 2006; 91:4022–4027. PMID: 16849419.18. Collins JF, Bai L, Ghishan FK. The SLC20 family of proteins: dual functions as sodium-phosphate cotransporters and viral receptors. Pflugers Arch. 2004; 447:647–652. PMID: 12759754.19. Kavanaugh MP, Miller DG, Zhang W, Law W, Kozak SL, Kabat D, et al. Cell-surface receptors for gibbon ape leukemia virus and amphotropic murine retrovirus are inducible sodium-dependent phosphate symporters. Proc Natl Acad Sci U S A. 1994; 91:7071–7075. PMID: 8041748.
Article20. Berndt TJ, Schiavi S, Kumar R. "Phosphatonins" and the regulation of phosphorus homeostasis. Am J Physiol Renal Physiol. 2005; 289:F1170–F1182. PMID: 16275744.
Article21. Miyamoto K, Ito M, Tatsumi S, Kuwahata M, Segawa H. New aspect of renal phosphate reabsorption: the type IIc sodium-dependent phosphate transporter. Am J Nephrol. 2007; 27:503–515. PMID: 17687185.
Article22. Thompson DL, Sabbagh Y, Tenenhouse HS, Roche PC, Drezner MK, Salisbury JL, et al. Ontogeny of Phex/PHEX protein expression in mouse embryo and subcellular localization in osteoblasts. J Bone Miner Res. 2002; 17:311–320. PMID: 11811562.
Article23. Brewer AJ, Canaff L, Hendy GN, Tenenhouse HS. Differential regulation of PHEX expression in bone and parathyroid gland by chronic renal insufficiency and 1,25-dihydroxyvitamin D3. Am J Physiol Renal Physiol. 2004; 286:F739–F748. PMID: 14693675.
Article24. Hruska KA, Rifas L, Cheng SL, Gupta A, Halstead L, Avioli L. X-linked hypophosphatemic rickets and the murine Hyp homologue. Am J Physiol. 1995; 268:F357–F362. PMID: 7900834.
Article25. Schiavi SC, Kumar R. The phosphatonin pathway: new insights in phosphate homeostasis. Kidney Int. 2004; 65:1–14. PMID: 14675031.
Article26. Perwad F, Azam N, Zhang MY, Yamashita T, Tenenhouse HS, Portale AA. Dietary and serum phosphorus regulate fibroblast growthfactor 23 expression and 1,25-dihydroxyvitamin D metabolism in mice. Endocrinology. 2005; 146:5358–5364. PMID: 16123154.27. Perwad F, Zhang MY, Tenenhouse HS, Portale AA. Fibroblast growth factor 23 impairs phosphorus and vitamin D metabolism in vivo and suppresses 25-hydroxyvitamin D-1alpha-hydroxylase expression in vitro. Am J Physiol Renal Physiol. 2007; 293:F1577–F1583. PMID: 17699549.28. Burnett SM, Gunawardene SC, Bringhurst FR, Juppner H, Lee H, Finkelstein JS. Regulation of C-terminal and intact FGF-23 by dietary phosphate in men and women. J Bone Miner Res. 2006; 21:1187–1196. PMID: 16869716.
Article29. Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, et al. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest. 2004; 113:561–568. PMID: 14966565.
Article30. Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, et al. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res. 2004; 19:429–435. PMID: 15040831.
Article31. Kolek OI, Hines ER, Jones MD, LeSueur LK, Lipko MA, Kiela PR, et al. 1alpha,25-Dihydroxyvitamin D3 upregulates FGF23 gene expression in bone: the final link in a renal-gastrointestinal-skeletal axis that controls phosphate transport. Am J Physiol Gastrointest Liver Physiol. 2005; 289:G1036–G1042. PMID: 16020653.32. Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, Goetz R, Kuro-o M, Mohammadi M, et al. The parathyroid is a target organ for FGF23 in rats. J Clin Invest. 2007; 117:4003–4008. PMID: 17992255.
Article33. Yamashita T, Konishi M, Miyake A, Inui K, Itoh N. Fibroblast growth factor (FGF)-23 inhibits renal phosphate reabsorption by activation of the mitogen-activated protein kinase pathway. J Biol Chem. 2002; 277:28265–28270. PMID: 12032146.34. Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006; 444:770–774. PMID: 17086194.
Article35. Berndt T, Craig TA, Bowe AE, Vassiliadis J, Reczek D, Finnegan R, et al. Secreted frizzled-related protein 4 is a potent tumor-derived phosphaturic agent. J Clin Invest. 2003; 112:785–794. PMID: 12952927.
Article36. Rowe PS, de Zoysa PA, Dong R, Wang HR, White KE, Econs MJ, et al. MEPE, a new gene expressed in bone marrow and tumors causing osteomalacia. Genomics. 2000; 67:54–68. PMID: 10945470.
Article37. Argiro L, Desbarats M, Glorieux FH, Ecarot B. Mepe, the gene encoding a tumor-secreted protein in oncogenic hypophosphatemic osteomalacia, is expressed in bone. Genomics. 2001; 74:342–351. PMID: 11414762.
Article38. Karim Z, Gerard B, Bakouh N, Alili R, Leroy C, Beck L, et al. NHERF1 mutations and responsiveness of renal parathyroid hormone. N Engl J Med. 2008; 359:1128–1135. PMID: 18784102.39. Hernando N, Gisler SM, Pribanic S, Deliot N, Capuano P, Wagner CA, et al. NaPi-IIa and interacting partners. J Physiol. 2005; 567:21–26. PMID: 15890704.
Article40. Villa-Bellosta R, Barac-Nieto M, Breusegem SY, Barry NP, Levi M, Sorribas V. Interactions of the growth-related, type IIc renal sodium/phosphate cotransporter with PDZ proteins. Kidney Int. 2008; 73:456–464. PMID: 18046316.
Article41. Khundmiri SJ, Ahmad A, Bennett RE, Weinman EJ, Steplock D, Cole J, et al. Novel regulatory function for NHERF-1 in Npt2a transcription. Am J Physiol Renal Physiol. 2008; 294:F840–F849. PMID: 18216150.
Article42. Francis F, Hennig S, Korn B, Reinhardt R, de Jong P, Poustka A, et al. A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. The HYP Consortium. Nat Genet. 1995; 11:130–136. PMID: 7550339.43. Holm IA, Huang X, Kunkel LM. Mutational analysis of the PEX gene in patients with X-linked hypophosphatemic rickets. Am J Hum Genet. 1997; 60:790–797. PMID: 9106524.44. Benet-Pages A, Lorenz-Depiereux B, Zischka H, White KE, Econs MJ, Strom TM. FGF23 is processed by proprotein convertases but not by PHEX. Bone. 2004; 35:455–462. PMID: 15268897.
Article46. Shimada T, Mizutani S, Muto T, Yoneya T, Hino R, Takeda S, et al. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci U S A. 2001; 98:6500–6505. PMID: 11344269.
Article47. Jonsson KB, Zahradnik R, Larsson T, White KE, Sugimoto T, Imanishi Y, et al. Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N Engl J Med. 2003; 348:1656–1663. PMID: 12711740.
Article48. Tenenhouse HS, Martel J, Gauthier C, Segawa H, Miyamoto K. Differential effects of Npt2a gene ablation and X-linked Hyp mutation on renal expression of Npt2c. Am J Physiol Renal Physiol. 2003; 285:F1271–F1278. PMID: 12952859.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Acute Phosphate Nephropathy after Sodium Phosphate Preparation
- A case of biopsy-proven chronic kidney disease on progression from acute phosphate nephropathy
- The Role of the Kidney in Glucose Metabolism
- Treatment of phosphate retention: The earlier the better?
- A Case of Chronic Renal Failure after Exposure to Oral Sodium Phosphate Bowel Purgatives