Blood Res.
2015 Dec;50(4):194-203. 10.5045/br.2015.50.4.194.
Hematopoietic stem cell expansion and generation: the ways to make a breakthrough
- Affiliations
-
- 1Department of Bioscience and Biotechnology, Sejong University, Korea. changkim@sejong.ac.kr
- 2Department of Pediatrics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Korea. hema2170@skku.edu
- 3Department of Medical Device Management and Research, SAIHST, Sungkyunkwan University, Seoul, Korea.
- KMID: 2133754
- DOI: http://doi.org/10.5045/br.2015.50.4.194
Abstract
- Hematopoietic stem cell transplantation (HSCT) is the first field where human stem cell therapy was successful. Flooding interest on human stem cell therapy to cure previously incurable diseases is largely indebted to HSCT success. Allogeneic HSCT has been an important modality to cure various diseases including hematologic malignancies, various non-malignant hematologic diseases, primary immunodeficiency diseases, and inborn errors of metabolism, while autologous HSCT is generally performed to rescue bone marrow aplasia following high-dose chemotherapy for solid tumors or multiple myeloma. Recently, HSCs are also spotlighted in the field of regenerative medicine for the amelioration of symptoms caused by neurodegenerative diseases, heart diseases, and others. Although the demand for HSCs has been growing, their supply often fails to meet the demand of the patients needing transplant due to a lack of histocompatible donors or a limited cell number. This review focuses on the generation and large-scale expansion of HSCs, which might overcome current limitations in the application of HSCs for clinical use. Furthermore, current proof of concept to replenish hematological homeostasis from non-hematological origin will be covered.
Keyword
MeSH Terms
Figure
Reference
-
1. Meuwissen HJ, Gatti RA, Terasaki PI, Hong R, Good RA. Treatment of lymphopenic hypogammaglobulinemia and bone-marrow aplasia by transplantation of allogeneic marrow. Crucial role of histocompatiility matching. N Engl J Med. 1969; 281:691–697. PMID: 4186068.2. Oran B, Shpall E. Umbilical cord blood transplantation: a maturing technology. Hematology Am Soc Hematol Educ Program. 2012; 2012:215–222. PMID: 23233584.
Article3. Pineault N, Abu-Khader A. Advances in umbilical cord blood stem cell expansion and clinical translation. Exp Hematol. 2015; 43:498–513. PMID: 25970610.
Article4. Scaradavou A, Brunstein CG, Eapen M, et al. Double unit grafts successfully extend the application of umbilical cord blood transplantation in adults with acute leukemia. Blood. 2013; 121:752–758. PMID: 23223509.
Article5. Barker JN, Weisdorf DJ, DeFor TE, et al. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. 2005; 105:1343–1347. PMID: 15466923.
Article6. Pasquini MC, Zhu X. Current uses and outcomes of hematopoietic stem cell transplantation: 2014 CIBMTR summary slides. Minneapolis, MN: Center for International Blood and Marrow Transplantation Research (CIBMTR);2015. Accessed October 11, 2015. https://www.cibmtr.org/ReferenceCenter/SlidesReports/SummarySlides/pages/index.aspx.7. de Lima M, McNiece I, Robinson SN, et al. Cord-blood engraftment with ex vivo mesenchymal-cell coculture. N Engl J Med. 2012; 367:2305–2315. PMID: 23234514.8. Horwitz ME, Chao NJ, Rizzieri DA, et al. Umbilical cord blood expansion with nicotinamide provides long-term multilineage engraftment. J Clin Invest. 2014; 124:3121–3128. PMID: 24911148.
Article9. Delaney C, Heimfeld S, Brashem-Stein C, Voorhies H, Manger RL, Bernstein ID. Notch-mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution. Nat Med. 2010; 16:232–236. PMID: 20081862.
Article10. Wagner JE, Brunstein C, McKenna D, et al. Acceleration of umbilical cord blood (UCB) stem engraftment: Results of a phase I clinical trial with stemregenin-1 (SR1) expansion culture. Biol Blood Marrow Transplant. 2015; 21:S48–S49.
Article11. Silva LC, Ortigosa LC, Benard G. Anti-TNF-α agents in the treatment of immune-mediated inflammatory diseases: mechanisms of action and pitfalls. Immunotherapy. 2010; 2:817–833. PMID: 21091114.
Article12. Elman JS, Li M, Wang F, Gimble JM, Parekkadan B. A comparison of adipose and bone marrow-derived mesenchymal stromal cell secreted factors in the treatment of systemic inflammation. J Inflamm (Lond). 2014; 11:1. PMID: 24397734.
Article13. Reardon S, Cyranoski D. Japan stem-cell trial stirs envy. Nature. 2014; 513:287–288. PMID: 25230622.
Article14. Petersdorf EW, Gooley TA, Anasetti C, et al. Optimizing outcome after unrelated marrow transplantation by comprehensive matching of HLA class I and II alleles in the donor and recipient. Blood. 1998; 92:3515–3520. PMID: 9808542.
Article15. Hansen JA, Gooley TA, Martin PJ, et al. Bone marrow transplants from unrelated donors for patients with chronic myeloid leukemia. N Engl J Med. 1998; 338:962–968. PMID: 9521984.
Article16. de Lima M, McMannis J, Gee A, et al. Transplantation of ex vivo expanded cord blood cells using the copper chelator tetraethylenepentamine: a phase I/II clinical trial. Bone Marrow Transplant. 2008; 41:771–778. PMID: 18209724.
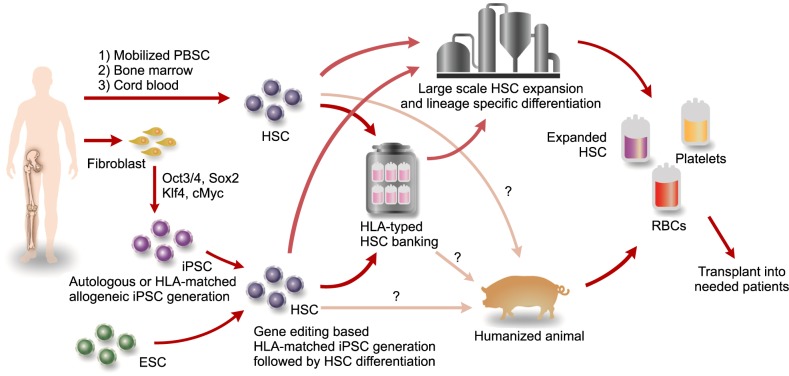
Article17. Taylor CJ, Peacock S, Chaudhry AN, Bradley JA, Bolton EM. Generating an iPSC bank for HLA-matched tissue transplantation based on known donor and recipient HLA types. Cell Stem Cell. 2012; 11:147–152. PMID: 22862941.
Article18. Wagner JE Jr, Eapen M, Carter S, Wang Y, et al. One-unit versus two-unit cord-blood transplantation for hematologic cancers. N Engl J Med. 2014; 371:1685–1694. PMID: 25354103.
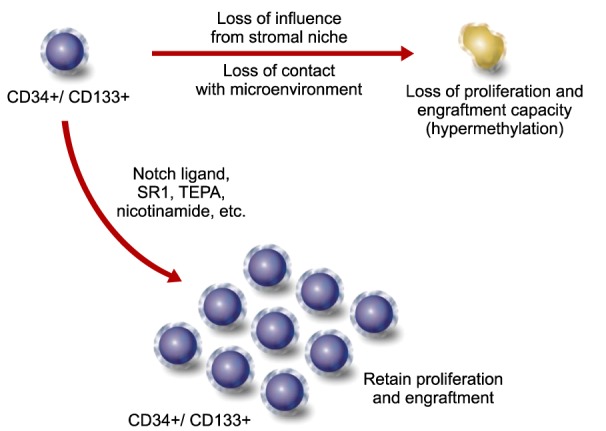
Article19. Weidner CI, Walenda T, Lin Q, et al. Hematopoietic stem and progenitor cells acquire distinct DNA-hypermethylation during in vitro culture. Sci Rep. 2013; 3:3372. PMID: 24284763.
Article20. Xie J, Zhang C. Ex vivo expansion of hematopoietic stem cells. Sci China Life Sci. 2015; 58:839–853. PMID: 26246379.
Article21. Chaurasia P, Gajzer DC, Schaniel C, D'Souza S, Hoffman R. Epigenetic reprogramming induces the expansion of cord blood stem cells. J Clin Invest. 2014; 124:2378–2395. PMID: 24762436.
Article22. Young JC, Wu S, Hansteen G, et al. Inhibitors of histone deacetylases promote hematopoietic stem cell self-renewal. Cytotherapy. 2004; 6:328–336. PMID: 16146885.
Article23. Araki H, Yoshinaga K, Boccuni P, Zhao Y, Hoffman R, Mahmud N. Chromatin-modifying agents permit human hematopoietic stem cells to undergo multiple cell divisions while retaining their repopulating potential. Blood. 2007; 109:3570–3578. PMID: 17185465.
Article24. Peled T, Shoham H, Aschengrau D, et al. Nicotinamide, a SIRT1 inhibitor, inhibits differentiation and facilitates expansion of hematopoietic progenitor cells with enhanced bone marrow homing and engraftment. Exp Hematol. 2012; 40:342–355.e1. PMID: 22198152.25. Lang J, Weiss N, Freed BM, Torres RM, Pelanda R. Generation of hematopoietic humanized mice in the newborn BALB/c-Rag2null Il2rγnull mouse model: a multivariable optimization approach. Clin Immunol. 2011; 140:102–116. PMID: 21536497.26. Berges BK, Wheat WH, Palmer BE, Connick E, Akkina R. HIV-1 infection and CD4 T cell depletion in the humanized Rag2-/-gamma c-/- (RAG-hu) mouse model. Retrovirology. 2006; 3:76. PMID: 17078891.
Article27. Matsuoka Y, Sumide K, Kawamura H, et al. Human cord blood-derived primitive CD34-negative hematopoietic stem cells (HSCs) are myeloid-biased long-term repopulating HSCs. Blood Cancer J. 2015; 5:e290. PMID: 25768404.
Article28. Tanner A, Taylor SE, Decottignies W, Berges BK. Humanized mice as a model to study human hematopoietic stem cell transplantation. Stem Cells Dev. 2014; 23:76–82. PMID: 23962058.
Article29. Brehm MA, Shultz LD, Luban J, Greiner DL. Overcoming current limitations in humanized mouse research. J Infect Dis. 2013; 208(Suppl 2):S125–S130. PMID: 24151318.
Article30. Lee K, Kwon DN, Ezashi T, et al. Engraftment of human iPS cells and allogeneic porcine cells into pigs with inactivated RAG2 and accompanying severe combined immunodeficiency. Proc Natl Acad Sci U S A. 2014; 111:7260–7265. PMID: 24799706.
Article31. Larochelle A, Vormoor J, Hanenberg H, et al. Identification of primitive human hematopoietic cells capable of repopulating NOD/SCID mouse bone marrow: implications for gene therapy. Nat Med. 1996; 2:1329–1337. PMID: 8946831.
Article32. Ishii M, Matsuoka Y, Sasaki Y, et al. Development of a high-resolution purification method for precise functional characterization of primitive human cord blood-derived CD34-negative SCID-repopulating cells. Exp Hematol. 2011; 39:203–213.e1. PMID: 21112372.
Article33. Lu R, Neff NF, Quake SR, Weissman IL. Tracking single hematopoietic stem cells in vivo using high-throughput sequencing in conjunction with viral genetic barcoding. Nat Biotechnol. 2011; 29:928–933. PMID: 21964413.
Article34. Kim C. iPSC technology-Powerful hand for disease modeling and therapeutic screen. BMB Rep. 2015; 48:256–265. PMID: 25104399.
Article35. Kim H, Kim JS. A guide to genome engineering with programmable nucleases. Nat Rev Genet. 2014; 15:321–334. PMID: 24690881.
Article36. Maeder ML, Linder SJ, Cascio VM, Fu Y, Ho QH, Joung JK. CRISPR RNA-guided activation of endogenous human genes. Nat Methods. 2013; 10:977–979. PMID: 23892898.
Article37. Kim C. Disease modeling and cell based therapy with iPSC: future therapeutic option with fast and safe application. Blood Res. 2014; 49:7–14. PMID: 24724061.
Article38. Ding Q, Lee YK, Schaefer EA, et al. A TALEN genome-editing system for generating human stem cell-based disease models. Cell Stem Cell. 2013; 12:238–251. PMID: 23246482.
Article39. Gaj T, Gersbach CA, Barbas CF 3rd. ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 2013; 31:397–405. PMID: 23664777.
Article40. Joung JK, Sander JD. TALENs: a widely applicable technology for targeted genome editing. Nat Rev Mol Cell Biol. 2013; 14:49–55. PMID: 23169466.
Article41. Schwartz SD, Hubschman JP, Heilwell G, et al. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet. 2012; 379:713–720. PMID: 22281388.
Article42. Song WK, Park KM, Kim HJ, et al. Treatment of macular degeneration using embryonic stem cell-derived retinal pigment epithelium: preliminary results in Asian patients. Stem Cell Reports. 2015; 4:860–872. PMID: 25937371.
Article43. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006; 126:663–676. PMID: 16904174.
Article44. Kanemura H, Go MJ, Shikamura M, et al. Tumorigenicity studies of induced pluripotent stem cell (iPSC)-derived retinal pigment epithelium (RPE) for the treatment of age-related macular degeneration. PLoS One. 2014; 9:e85336. PMID: 24454843.
Article45. Schlaeger TM, Daheron L, Brickler TR, et al. A comparison of non-integrating reprogramming methods. Nat Biotechnol. 2015; 33:58–63. PMID: 25437882.
Article46. Geron Corporation. World's first clinical trial of human embryonic stem cell therapy cleared. Regen Med. 2009; 4:161. PMID: 19322956.47. Hanna J, Wernig M, Markoulaki S, et al. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science. 2007; 318:1920–1923. PMID: 18063756.
Article48. Wang L, Li L, Menendez P, Cerdan C, Bhatia M. Human embryonic stem cells maintained in the absence of mouse embryonic fibroblasts or conditioned media are capable of hematopoietic development. Blood. 2005; 105:4598–4603. PMID: 15718421.
Article49. Woll PS, Grzywacz B, Tian X, et al. Human embryonic stem cells differentiate into a homogeneous population of natural killer cells with potent in vivo antitumor activity. Blood. 2009; 113:6094–6101. PMID: 19365083.
Article50. Galic Z, Kitchen SG, Kacena A, et al. T lineage differentiation from human embryonic stem cells. Proc Natl Acad Sci U S A. 2006; 103:11742–11747. PMID: 16844782.
Article51. Carpenter L, Malladi R, Yang CT, et al. Human induced pluripotent stem cells are capable of B-cell lymphopoiesis. Blood. 2011; 117:4008–4011. PMID: 21343609.
Article52. Knorr DA, Ni Z, Hermanson D, et al. Clinical-scale derivation of natural killer cells from human pluripotent stem cells for cancer therapy. Stem Cells Transl Med. 2013; 2:274–283. PMID: 23515118.
Article53. Ledran MH, Krassowska A, Armstrong L, et al. Efficient hematopoietic differentiation of human embryonic stem cells on stromal cells derived from hematopoietic niches. Cell Stem Cell. 2008; 3:85–98. PMID: 18593561.
Article54. Gori JL, Butler JM, Chan YY, et al. Vascular niche promotes hematopoietic multipotent progenitor formation from pluripotent stem cells. J Clin Invest. 2015; 125:1243–1254. PMID: 25664855.
Article55. Ramos-Mejía V, Navarro-Montero O, Ayllón V, et al. HOXA9 promotes hematopoietic commitment of human embryonic stem cells. Blood. 2014; 124:3065–3075. PMID: 25185710.
Article56. Real PJ, Ligero G, Ayllon V, et al. SCL/TAL1 regulates hematopoietic specification from human embryonic stem cells. Mol Ther. 2012; 20:1443–1453. PMID: 22491213.
Article57. Doulatov S, Vo LT, Chou SS, et al. Induction of multipotential hematopoietic progenitors from human pluripotent stem cells via respecification of lineage-restricted precursors. Cell Stem Cell. 2013; 13:459–470. PMID: 24094326.
Article58. Ran D, Shia WJ, Lo MC, et al. RUNX1a enhances hematopoietic lineage commitment from human embryonic stem cells and inducible pluripotent stem cells. Blood. 2013; 121:2882–2890. PMID: 23372166.
Article59. Amabile G, Welner RS, Nombela-Arrieta C, et al. In vivo generation of transplantable human hematopoietic cells from induced pluripotent stem cells. Blood. 2013; 121:1255–1264. PMID: 23212524.
Article60. Suzuki N, Yamazaki S, Yamaguchi T, et al. Generation of engraftable hematopoietic stem cells from induced pluripotent stem cells by way of teratoma formation. Mol Ther. 2013; 21:1424–1431. PMID: 23670574.
Article61. Szabo E, Rampalli S, Risueño RM, et al. Direct conversion of human fibroblasts to multilineage blood progenitors. Nature. 2010; 468:521–526. PMID: 21057492.
Article62. Sandler VM, Lis R, Liu Y, et al. Reprogramming human endothelial cells to haematopoietic cells requires vascular induction. Nature. 2014; 511:312–318. PMID: 25030167.
Article63. Riddell J, Gazit R, Garrison BS, et al. Reprogramming committed murine blood cells to induced hematopoietic stem cells with defined factors. Cell. 2014; 157:549–564. PMID: 24766805.
Article64. Batta K, Florkowska M, Kouskoff V, Lacaud G. Direct reprogramming of murine fibroblasts to hematopoietic progenitor cells. Cell Rep. 2014; 9:1871–1884. PMID: 25466247.
Article65. Lu SJ, Li F, Yin H, et al. Platelets generated from human embryonic stem cells are functional in vitro and in the microcirculation of living mice. Cell Res. 2011; 21:530–545. PMID: 21221130.
Article66. Nakamura S, Takayama N, Hirata S, et al. Expandable megakaryocyte cell lines enable clinically applicable generation of platelets from human induced pluripotent stem cells. Cell Stem Cell. 2014; 14:535–548. PMID: 24529595.
Article67. Kennedy M, Awong G, Sturgeon CM, et al. T lymphocyte potential marks the emergence of definitive hematopoietic progenitors in human pluripotent stem cell differentiation cultures. Cell Rep. 2012; 2:1722–1735. PMID: 23219550.
Article68. Liu Z, Lu SJ, Lu Y, et al. Transdifferentiation of Human Hair Follicle Mesenchymal Stem Cells into Red Blood Cells by OCT4. Stem Cells Int. 2015; 2015:389628. PMID: 25755671.
Article69. Vodyanik MA, Bork JA, Thomson JA, Slukvin II. Human embryonic stem cell-derived CD34+ cells: efficient production in the coculture with OP9 stromal cells and analysis of lymphohematopoietic potential. Blood. 2005; 105:617–626. PMID: 15374881.
Article70. Wada H, Kojo S, Kusama C, et al. Successful differentiation to T cells, but unsuccessful B-cell generation, from B-cell-derived induced pluripotent stem cells. Int Immunol. 2011; 23:65–74. PMID: 21135032.
Article71. Sturgeon CM, Ditadi A, Awong G, Kennedy M, Keller G. Wnt signaling controls the specification of definitive and primitive hematopoiesis from human pluripotent stem cells. Nat Biotechnol. 2014; 32:554–561. PMID: 24837661.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hematopoietic Stem Cells Culture, Expansion and Differentiation: An Insight into Variable and Available Media
- Hematopoietic Stem Cell Transplantation
- Opening the era of in vivo xenotransplantation model for hematopoietic stem cell transplantation
- In utero hematopoietic stem cell therapy
- Hematopoietic Stem Cell Transplantation in Inborn Error of Metabolism