Anat Cell Biol.
2015 Dec;48(4):244-250. 10.5115/acb.2015.48.4.244.
A combinational effect of acetaminophen and oriental herbs on the regulation of inflammatory mediators in microglia cell line, BV2
- Affiliations
-
- 1Department of Anatomy, Seoul National University College of Medicine, Seoul, Korea. genius29@snu.ac.kr
- 2Institute of Allergy and Clinical Immunology, Seoul National University Medical Research Center, Seoul, Korea.
- 3Department of Family Medicine, Eulji University School of Medicine, Daejeon, Korea.
- KMID: 2133263
- DOI: http://doi.org/10.5115/acb.2015.48.4.244
Abstract
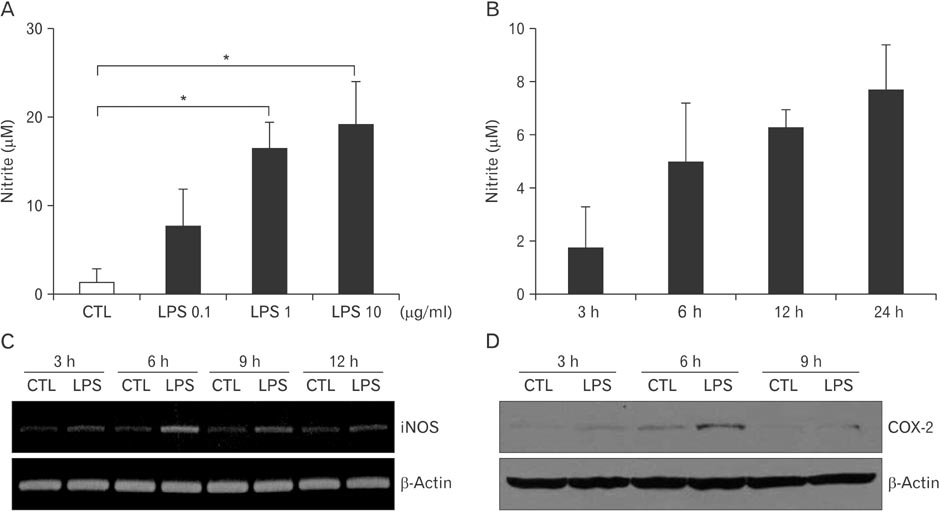
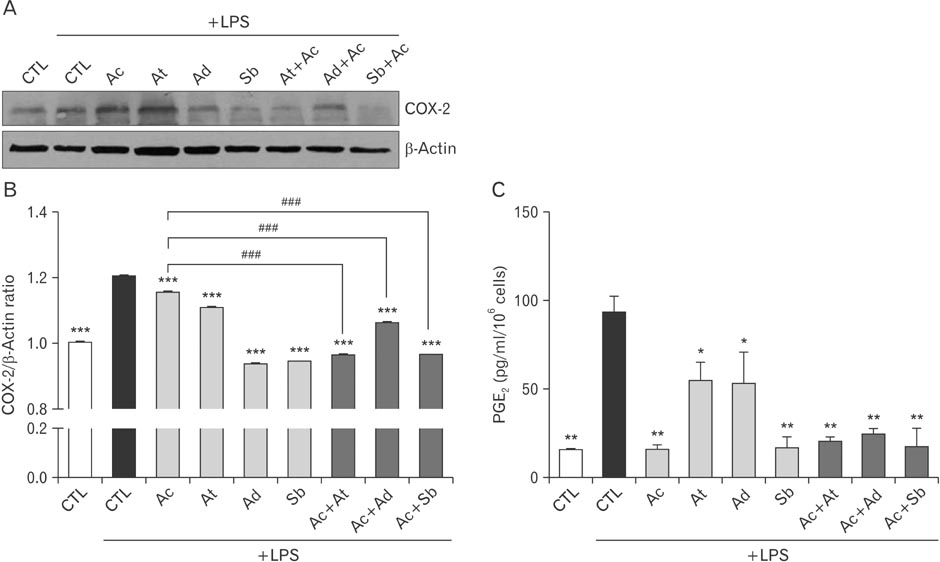
- The mechanism of Western medicine that is commonly used for pain relief is well-known. However, very little is known for oriental herbs, and even less is known for mixture of the two. We investigated the combinational effect of 3 kinds of oriental herbs, usually used for the control of headache, and acetaminophen to relieve headache in microglia cell line, BV2. Lipopolysaccharide (LPS) stimulation induced to produce nitrite and increased the expression of inflammation-related factors like inducible nitric oxide synthase and cyclooxygenase-2 (COX-2) in murine microglia cell line, BV2. Oriental herbs such as Angelica tenuissima, Angelica dahurica, and Scutellaria baicalensis reduced the production of nitric oxide and the expression of COX-2. Moreover, a treatment of acetaminophen combined with oriental herbs was more decreased the COX-2 expression, and its product, prostaglandin E2 production in BV2 cells. Therefore, a combined treatment of oriental herbs such as A. tenuissima, A. dahurica, and S. baicalensis and Western medicine like acetaminophen has a synergistic effect on the decrease of LPS-induced inflammation in microglia.
Keyword
MeSH Terms
Figure
Reference
-
1. Graham GG, Davies MJ, Day RO, Mohamudally A, Scott KF. The modern pharmacology of paracetamol: therapeutic actions, mechanism of action, metabolism, toxicity and recent pharmacological findings. Inflammopharmacology. 2013; 21:201–232.2. Bisaglia M, Venezia V, Piccioli P, Stanzione S, Porcile C, Russo C, Mancini F, Milanese C, Schettini G. Acetaminophen protects hippocampal neurons and PC12 cultures from amyloid beta-peptides induced oxidative stress and reduces NF-kappaB activation. Neurochem Int. 2002; 41:43–54.3. Locke CJ, Fox SA, Caldwell GA, Caldwell KA. Acetaminophen attenuates dopamine neuron degeneration in animal models of Parkinson's disease. Neurosci Lett. 2008; 439:129–133.4. Tripathy D, Grammas P. Acetaminophen protects brain endothelial cells against oxidative stress. Microvasc Res. 2009; 77:289–296.5. Kim EH, Shim B, Kang S, Jeong G, Lee JS, Yu YB, Chun M. Anti-inflammatory effects of Scutellaria baicalensis extract via suppression of immune modulators and MAP kinase signaling molecules. J Ethnopharmacol. 2009; 126:320–331.6. Yoon SB, Lee YJ, Park SK, Kim HC, Bae H, Kim HM, Ko SG, Choi HY, Oh MS, Park W. Anti-inflammatory effects of Scutellaria baicalensis water extract on LPS-activated RAW 264.7 macrophages. J Ethnopharmacol. 2009; 125:286–290.7. Gasiorowski K, Lamer-Zarawska E, Leszek J, Parvathaneni K, Yendluri BB, Blach-Olszewska Z, Aliev G. Flavones from root of Scutellaria baicalensis Georgi: drugs of the future in neurodegeneration? CNS Neurol Disord Drug Targets. 2011; 10:184–191.8. Lee SY, Park HJ, Cha DS, Shin TY, Na HJ, Moon WS, Kang YG, Jeon H. Antioxidant and anti-inflammatory effect of Angelica tenuissima in IFN-γ/LPS-stimulated peritoneal macrophage. Korean J Orient Physiol Pathol. 2008; 22:1549–1556.9. Lee MY, Lee JA, Seo CS, Ha H, Lee H, Son JK, Shin HK. Anti-inflammatory activity of Angelica dahurica ethanolic extract on RAW264.7 cells via upregulation of heme oxygenase-1. Food Chem Toxicol. 2011; 49:1047–1055.10. Ross J, Auger M, Burke B, Lewis C. The biology of the macrophage. Macrophage. 2002; 2:16–23.11. Gao HM, Hong JS. Why neurodegenerative diseases are progressive: uncontrolled inflammation drives disease progression. Trends Immunol. 2008; 29:357–365.12. Krause DL, Müller N. Neuroinflammation, microglia and implications for anti-inflammatory treatment in Alzheimer's disease. Int J Alzheimers Dis. 2010; 2010:732806.13. Hutchinson MR, Bland ST, Johnson KW, Rice KC, Maier SF, Watkins LR. Opioid-induced glial activation: mechanisms of activation and implications for opioid analgesia, dependence, and reward. ScientificWorldJournal. 2007; 7:98–111.14. Bartley J. Could glial activation be a factor in migraine? Med Hypotheses. 2009; 72:255–257.15. Hinz B, Cheremina O, Brune K. Acetaminophen (paracetamol) is a selective cyclooxygenase-2 inhibitor in man. FASEB J. 2008; 22:383–390.16. Giuliano F, Warner TD. Origins of prostaglandin E2: involvements of cyclooxygenase (COX)-1 and COX-2 in human and rat systems. J Pharmacol Exp Ther. 2002; 303:1001–1006.17. Waeber C, Moskowitz MA. Migraine as an inflammatory disorder. Neurology. 2005; 64:10 Suppl 2. S9–S15.18. Watkins LR, Milligan ED, Maier SF. Glial proinflammatory cytokines mediate exaggerated pain states: implications for clinical pain. Adv Exp Med Biol. 2003; 521:1–21.19. Diener HC, Katsarava Z, Limmroth V. Current diagnosis and treatment of migraine. Ophthalmologe. 2008; 105:501–508.20. Rasmussen BK, Jensen R, Schroll M, Olesen J. Epidemiology of headache in a general population: a prevalence study. J Clin Epidemiol. 1991; 44:1147–1157.21. Mett A, Tfelt-Hansen P. Acute migraine therapy: recent evidence from randomized comparative trials. Curr Opin Neurol. 2008; 21:331–337.22. Tzeng SF, Hsiao HY, Mak OT. Prostaglandins and cyclooxygenases in glial cells during brain inflammation. Curr Drug Targets Inflamm Allergy. 2005; 4:335–340.23. Findlay VJ, Tapiero H, Townsend DM. Sulfiredoxin: a potential therapeutic agent? Biomed Pharmacother. 2005; 59:374–379.24. Buhrmann C, Shayan P, Aggarwal BB, Shakibaei M. Evidence that TNF-beta (lymphotoxin alpha) can activate the inflammatory environment in human chondrocytes. Arthritis Res Ther. 2013; 15:R202.25. Franklin CC, Backos DS, Mohar I, White CC, Forman HJ, Kavanagh TJ. Structure, function, and post-translational regulation of the catalytic and modifier subunits of glutamate cysteine ligase. Mol Aspects Med. 2009; 30:86–98.26. Uttara B, Singh AV, Zamboni P, Mahajan RT. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol. 2009; 7:65–74.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Regulatory Effect of 25-hydroxyvitamin D3 on Nitric Oxide Production in Activated Microglia
- Interaction of HIV-1 core p24 antigen with human monocytic cell line THP1 results in TNF-alpha dependent secretion of matrix metalloproteinase-9
- Effects of Seongpungtang on the Nitric oxide Production of C(6) glial cell and Microglia
- Activation of Autophagy Pathway Suppresses the Expression of iNOS, IL6 and Cell Death of LPS-Stimulated Microglia Cells
- A New Neolignan Derivative, Balanophonin Isolated from Firmiana simplex Delays the Progress of Neuronal Cell Death by Inhibiting Microglial Activation