J Korean Med Sci.
2014 Oct;29(10):1385-1390. 10.3346/jkms.2014.29.10.1385.
Association between Adipokines and Coronary Artery Lesions in Children with Kawasaki Disease
- Affiliations
-
- 1Department of Pediatrics, Eulji Universitiy School of Medicine, Daejeon, Korea.
- 2Eulji Medi-Bio Research Institute, Eulji University, Daejeon, Korea.
- 3Department of Pediatrics, Chungnam National University School of Medicine, Daejeon, Korea. gilhong@cnu.ac.kr
- KMID: 2129629
- DOI: http://doi.org/10.3346/jkms.2014.29.10.1385
Abstract
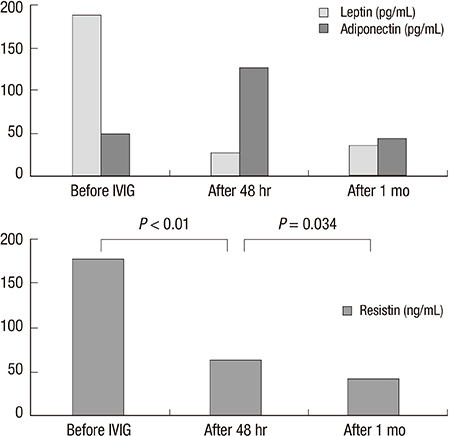
- Body fat is an important source of adipokine, which is associated with energy balance and inflammatory and immune responses. However, the role of adipokines in coronary artery complications in Kawasaki disease (KD) has not yet been fully explained. We investigated whether serum adipokine level can be a useful marker for patients with KD who are at higher risk of developing coronary artery lesion (CAL). We measured adipokine levels and other inflammatory parameters in 40 patients with KD, 32 febrile controls, and 15 afebrile controls. Interleukin (IL)-6, tumor necrosis factor (TNF)-alpha and other laboratory parameters were also measured before and after intravenous immunoglobulin therapy, and in the convalescent phase. At admission, the serum resistin levels in KD children were significantly higher than those in controls (177.56 ng/mL in KD children, 76.48 ng/mL in febrile controls, and 17.95 ng/mL in afebrile controls). In patients with KD, resistin levels were significantly associated with decreased hemoglobin levels (P=0.049) and increased IL-6 levels (P=0.014). The serum IL-6 levels were significantly higher and body mass index was significantly lower in the group of KD with CALs than those without CALs (228.26 ng/mL vs. 39.18 ng/mL and 15.09 vs. 16.60, respectively). In conclusion, resistin is significantly elevated in KD patients, although it has no prognostic value of predicting coronary artery lesion in the acute stage.
Keyword
MeSH Terms
-
Biological Markers/*blood
Child
Child, Preschool
Coronary Vessels/pathology
Echocardiography
Female
Hemoglobins/analysis
Humans
Immunoglobulins, Intravenous/therapeutic use
Inflammation/blood/immunology
Interleukin-6/*blood
Male
Mucocutaneous Lymph Node Syndrome/*blood/pathology
Resistin/*blood
Tumor Necrosis Factor-alpha/*blood
Biological Markers
Hemoglobins
Immunoglobulins, Intravenous
Interleukin-6
Resistin
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Matsubara T, Ichiyama T, Furukawa S. Immunological profile of peripheral blood lymphocytes and monocytes/macrophages in Kawasaki disease. Clin Exp Immunol. 2005; 141:381–387.2. Lago F, Dieguez C, Gómez-Reino J, Gualillo O. The emerging role of adipokines as mediators of inflammation and immune responses. Cytokine Growth Factor Rev. 2007; 18:313–325.3. Takeshita S, Takabayashi H, Yoshida N. Circulating adiponectin levels in Kawasaki disease. Acta Paediatr. 2006; 95:1312–1314.4. Nozue H, Imai H, Saitoh H, Aoki T, Ichikawa K, Kamoda T. Serum resistin concentrations in children with Kawasaki disease. Inflamm Res. 2010; 59:915–920.5. Shamsuzzaman AS, Winnicki M, Wolk R, Svatikova A, Phillips BG, Davison DE, Berger PB, Somers VK. Independent association between plasma leptin and C-reactive protein in healthy humans. Circulation. 2004; 109:2181–2185.6. Wallace AM, McMahon AD, Packard CJ, Kelly A, Shepherd J, Gaw A, Sattar N. Plasma leptin and the risk of cardiovascular disease in the west of Scotland coronary prevention study (WOSCOPS). Circulation. 2001; 104:3052–3056.7. Liu R, He B, Gao F, Liu Q, Yi Q. Relationship between adipokines and coronary artery aneurysm in children with Kawasaki disease. Transl Res. 2012; 160:131–136.8. McCrindle BW, Li JS, Minich LL, Colan SD, Atz AM, Takahashi M, Vetter VL, Gersony WM, Mitchell PD, Newburger JW. Coronary artery involvement in children with Kawasaki disease: risk factors from analysis of serial normalized measurements. Circulation. 2007; 116:174–179.9. Haycock GB, Schwartz GJ, Wisotsky DH. Geometric method for measuring body surface area: a height-weight formula validated in infants, children, and adults. J Pediatr. 1978; 93:62–66.10. Toussirot E, Streit G, Wendling D. The contribution of adipose tissue and adipokines to inflammation in joint diseases. Curr Med Chem. 2007; 14:1095–1100.11. Sanna V, Di Giacomo A, La Cava A, Lechler RI, Fontana S, Zappacosta S, Matarese G. Leptin surge precedes onset of autoimmune encephalomyelitis and correlates with development of pathogenic T cell responses. J Clin Invest. 2003; 111:241–250.12. Singhal A, Farooqi IS, Cole TJ, O'Rahilly S, Fewtrell M, Kattenhorn M, Lucas A, Deanfield J. Influence of leptin on arterial distensibility: a novel link between obesity and cardiovascular disease? Circulation. 2002; 106:1919–1924.13. Takemura Y, Ouchi N, Shibata R, Aprahamian T, Kirber MT, Summer RS, Kihara S, Walsh K. Adiponectin modulates inflammatory reactions via calreticulin receptor-dependent clearance of early apoptotic bodies. J Clin Invest. 2007; 117:375–386.14. Fasshauer M, Kralisch S, Klier M, Lossner U, Bluher M, Klein J, Paschke R. Adiponectin gene expression and secretion is inhibited by interleukin-6 in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2003; 301:1045–1050.15. Ouchi N, Kihara S, Arita Y, Maeda K, Kuriyama H, Okamoto Y, Hotta K, Nishida M, Takahashi M, Nakamura T, et al. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation. 1999; 100:2473–2476.16. Hopkins TA, Ouchi N, Shibata R, Walsh K. Adiponectin actions in the cardiovascular system. Cardiovasc Res. 2007; 74:11–18.17. Shibata R, Ouchi N, Murohara T. Adiponectin and cardiovascular disease. Circ J. 2009; 73:608–614.18. Neumeier M, Weigert J, Schäffler A, Wehrwein G, Müller-Ladner U, Schölmerich J, Wrede C, Buechler C. Different effects of adiponectin isoforms in human monocytic cells. J Leukoc Biol. 2006; 79:803–808.19. Nagaev I, Smith U. Insulin resistance and type 2 diabetes are not related to resistin expression in human fat cells or skeletal muscle. Biochem Biophys Res Commun. 2001; 285:561–564.20. Migita K, Maeda Y, Miyashita T, Kimura H, Nakamura M, Ishibashi H, Eguchi K. The serum levels of resistin in rheumatoid arthritis patients. Clin Exp Rheumatol. 2006; 24:698–701.21. Konrad A, Lehrke M, Schachinger V, Seibold F, Stark R, Ochsenkühn T, Parhofer KG, Göke B, Broedl UC. Resistin is an inflammatory marker of inflammatory bowel disease in humans. Eur J Gastroenterol Hepatol. 2007; 19:1070–1074.22. Larochelle J, Freiler J, Dice J, Hagan L. Plasma resistin levels in asthmatics as a marker of disease state. J Asthma. 2007; 44:509–513.23. Gabay C. Interleukin-6 and chronic inflammation. Arthritis Res Ther. 2006; 8:S3.24. Lin CY, Lin CC, Hwang B, Chiang B. Serial changes of serum interleukin-6, interleukin-8, and tumor necrosis factor alpha among patients with Kawasaki disease. J Pediatr. 1992; 121:924–926.25. Wirth A, Ritthaler F, Roth A, Schlierf G. Reduced uptake and esterification of free fatty acids during prolonged fasting. Int J Obes. 1983; 7:353–359.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- CABG for an Adult with Coronary Disease due to Kawasaki Disease
- Laboratory Values in Patients with Kawasaki Disease after Intravenous Immunoglobulin: Comparison of Patients with Coronary Artery Lesions to those without Coronary Artery Lesions
- Percutaneous Transluminal Coronary Angioplasty for Coronary Artery Stenosis in an Adult Kawasaki Disease with Coronary Aneurysm : A Case Report and Review
- Changes in Coronary Perfusion after Occlusion of Coronary Arteries in Kawasaki Disease
- Predictive indicators of coronary artery complications in Kawasaki disease