J Korean Surg Soc.
2011 Aug;81(2):85-95. 10.4174/jkss.2011.81.2.85.
Induction of apoptosis with diallyl disulfide in AGS gastric cancer cell line
- Affiliations
-
- 1Department of Surgery, Hansol Hospital, Seoul, Korea.
- 2Department of Surgery, Ewha Womans University School of Medicine, Seoul, Korea. gsljh@ewha.ac.kr
- KMID: 2096661
- DOI: http://doi.org/10.4174/jkss.2011.81.2.85
Abstract
- PURPOSE
Diallyl disulfide (DADS) is a major organosulfur compound derived from garlic. It has been reported that DADS is able to inhibit the proliferation of several tumor cells. In this study, the effect of DADS was investigated in terms of the proliferation of AGS, gastric adenocarcinoma cell line at various concentrations.
METHODS
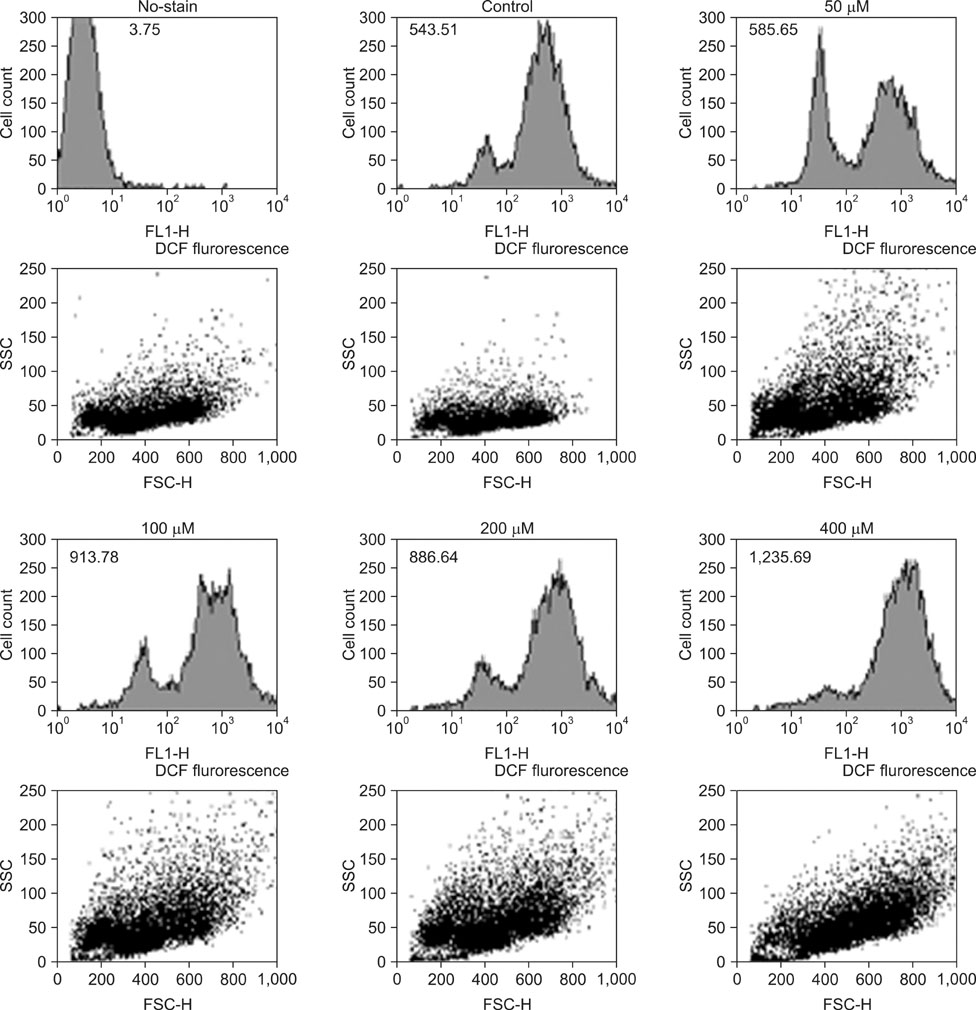
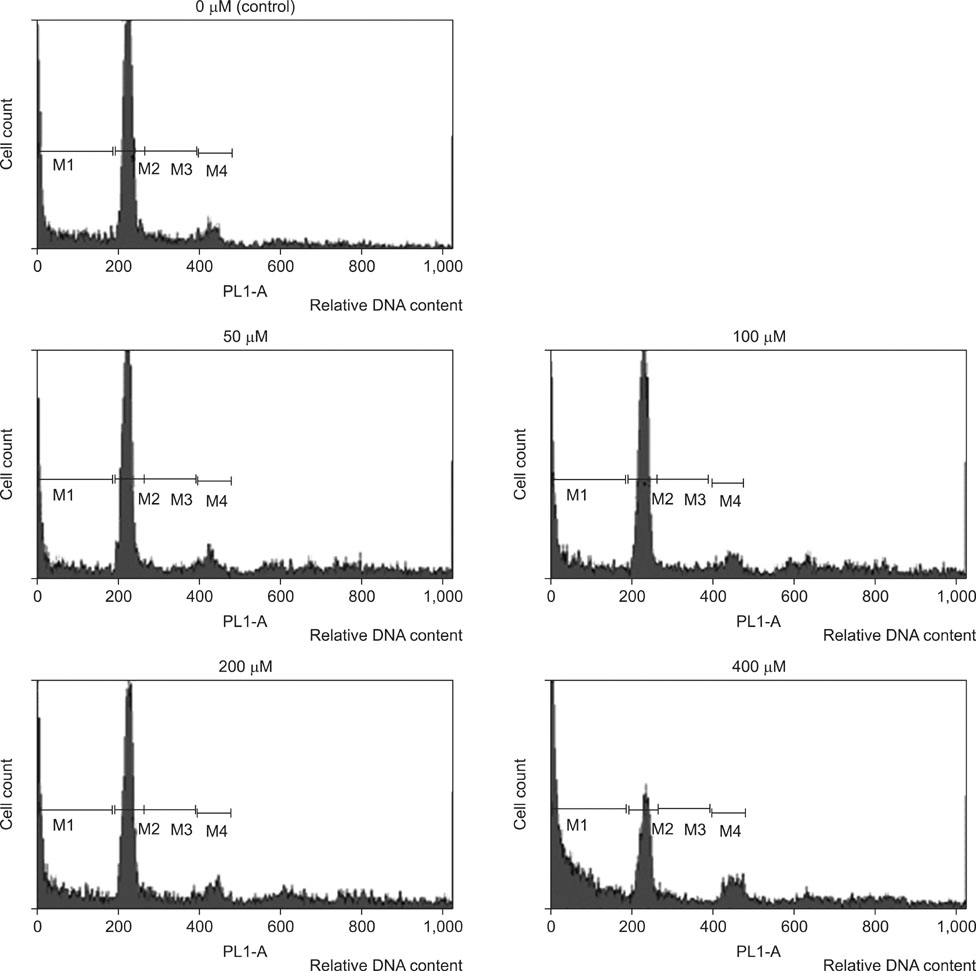
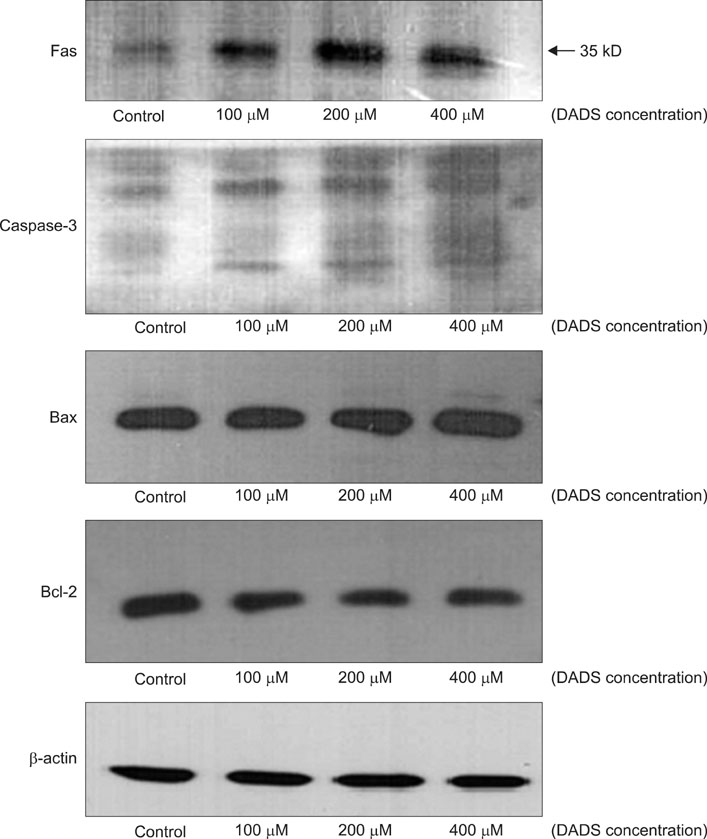
The viability of cultured cells was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide assay. To detect the induction of apoptosis, Annexin V-FITC/propodium iodide (PI) staining assay was performed. Analysis of reactive oxygen species (ROS) and the distribution of cells in the cell cycle were measured by a flow cytometer. And using the Western blot analysis, the change of Fas, caspase-3, Bax, Bcl-2 activity was measured.
RESULTS
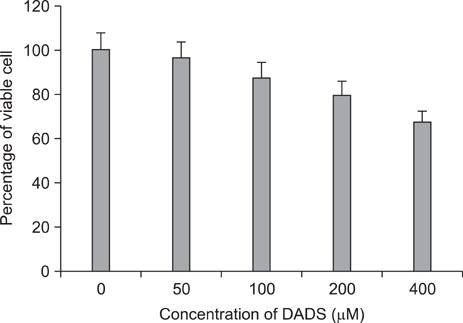
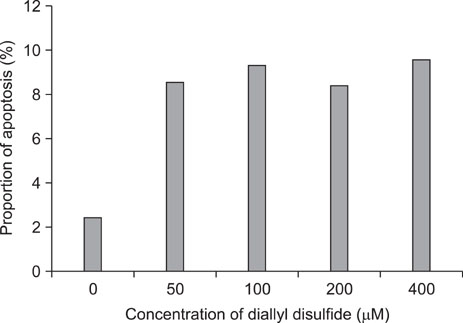
The percentage of live AGS cells was decreased to 23% of that in the control group after 400 microM DADS treatment for 48 hours. The Annexin V positive/PI negative (apoptosis portion) area increased from low concentration of DADS to high concentration. When comparing among the DADS treatment groups, the amount of ROS production increased in a dose dependent manner. The percentage of sub-diploid DNA content increased from 8.71% at 50 microM to 25.74% at 400 microM DADS treatment group. The expressions of Fas, caspase-3, Bax were increased and that of Bcl-2 was decreased in a dose dependent manner.
CONCLUSION
DADS decreases the viability of AGS cell lines and induces apoptosis in a dose-dependent manner. But the relationship of the anti-proliferative effect of DADS and related molecular changes were not clearly proportional to the concentration of DADS.
MeSH Terms
-
4-Acetamido-4'-isothiocyanatostilbene-2,2'-disulfonic Acid
Adenocarcinoma
Allyl Compounds
Annexin A5
Apoptosis
Blotting, Western
Caspase 3
Cell Cycle
Cell Line
Cells, Cultured
Disulfides
DNA
Garlic
Reactive Oxygen Species
Stomach Neoplasms
4-Acetamido-4'-isothiocyanatostilbene-2,2'-disulfonic Acid
Allyl Compounds
Annexin A5
Caspase 3
DNA
Disulfides
Reactive Oxygen Species
Figure
Reference
-
1. Sun SY, Hail N Jr, Lotan R. Apoptosis as a novel target for cancer chemoprevention. J Natl Cancer Inst. 2004. 96:662–672.2. Fleischauer AT, Arab L. Garlic and cancer: a critical review of the epidemiologic literature. J Nutr. 2001. 131:3S. 1032S–1040S.3. Wargovich MJ, Uda N, Woods C, Velasco M, McKee K. Allium vegetables: their role in the prevention of cancer. Biochem Soc Trans. 1996. 24:811–814.4. Milner JA. A historical perspective on garlic and cancer. J Nutr. 2001. 131:3S. 1027S–1031S.5. Khanum F, Anilakumar KR, Viswanathan KR. Anticarcinogenic properties of garlic: a review. Crit Rev Food Sci Nutr. 2004. 44:479–488.6. Lawson LD, Wang ZJ. Pre-hepatic fate of the organosulfur compounds derived from garlic (Allium sativum). Planta Med. 1993. 59:A688–A689.7. Bose C, Guo J, Zimniak L, Srivastava SK, Singh SP, Zimniak P, et al. Critical role of allyl groups and disulfide chain in induction of Pi class glutathione transferase in mouse tissues in vivo by diallyl disulfide, a naturally occurring chemopreventive agent in garlic. Carcinogenesis. 2002. 23:1661–1665.8. Filomeni G, Aquilano K, Rotilio G, Ciriolo MR. Reactive oxygen species-dependent c-Jun NH2-terminal kinase/ c-Jun signaling cascade mediates neuroblastoma cell death induced by diallyl disulfide. Cancer Res. 2003. 63:5940–5949.9. Wu XJ, Kassie F, Mersch-Sundermann V. The role of reactive oxygen species (ROS) production on diallyl disulfide (DADS) induced apoptosis and cell cycle arrest in human A549 lung carcinoma cells. Mutat Res. 2005. 579:115–124.10. Kwon KB, Yoo SJ, Ryu DG, Yang JY, Rho HW, Kim JS, et al. Induction of apoptosis by diallyl disulfide through activation of caspase-3 in human leukemia HL-60 cells. Biochem Pharmacol. 2002. 63:41–47.11. Nakagawa H, Tsuta K, Kiuchi K, Senzaki H, Tanaka K, Hioki K, et al. Growth inhibitory effects of diallyl disulfide on human breast cancer cell lines. Carcinogenesis. 2001. 22:891–897.12. Hong YS, Ham YA, Choi JH, Kim J. Effects of allyl sulfur compounds and garlic extract on the expression of Bcl-2, Bax, and p53 in non small cell lung cancer cell lines. Exp Mol Med. 2000. 32:127–134.13. Abraham KO, Shankaranarayana ML, Raghavan B, Nataraja CP. Alliums-varieties, chemistry, and analysis. Lebenson-Wiss U Technol. 1976. 9:193–200.14. You WC, Blot WJ, Chang YS, Ershow A, Yang ZT, An Q, et al. Allium vegetables and reduced risk of stomach cancer. J Natl Cancer Inst. 1989. 81:162–164.15. Song JD, Lee SK, Kim KM, Park SE, Park SJ, Kim KH, et al. Molecular mechanism of diallyl disulfide in cell cycle arrest and apoptosis in HCT-116 colon cancer cells. J Biochem Mol Toxicol. 2009. 23:71–79.16. Wan X, Polyakova Y, Row KH. Determination of diallyl disulfide in garlic by reversed-phase high performance liquid chromatography. Anal Sci Technol. 2007. 20:442–447.17. Hong WK, Sporn MB. Recent advances in chemoprevention of cancer. Science. 1997. 278:1073–1077.18. Omkumar RV, Kadam SM, Banerji A, Ramasarma T. On the involvement of intramolecular protein disulfide in the irreversible inactivation of 3-hydroxy-3-methylglutaryl-CoA reductase by diallyl disulfide. Biochim Biophys Acta. 1993. 1164:108–112.19. Oez S, Platzer E, Welte K. A quantitative colorimetric method to evaluate the functional state of human polymorphonuclear leukocytes. Blut. 1990. 60:97–102.20. Hengartner MO. The biochemistry of apoptosis. Nature. 2000. 407:770–776.21. Wang X, Martindale JL, Liu Y, Holbrook NJ. The cellular response to oxidative stress: influences of mitogen-activated protein kinase signalling pathways on cell survival. Biochem J. 1998. 333(Pt 2):291–300.22. Jacobson MD, Weil M, Raff MC. Programmed cell death in animal development. Cell. 1997. 88:347–354.23. Carmody RJ, Cotter TG. Signalling apoptosis: a radical approach. Redox Rep. 2001. 6:77–90.24. Peter ME, Krammer PH. The CD95(APO-1/Fas) DISC and beyond. Cell Death Differ. 2003. 10:26–35.25. Zou H, Henzel WJ, Liu X, Lutschg A, Wang X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell. 1997. 90:405–413.26. Ruvolo PP, Deng X, May WS. Phosphorylation of Bcl2 and regulation of apoptosis. Leukemia. 2001. 15:515–522.27. Filomeni G, Aquilano K, Rotilio G, Ciriolo MR. Glutathione-related systems and modulation of extracellular signal-regulated kinases are involved in the resistance of AGS adenocarcinoma gastric cells to diallyl disulfide-induced apoptosis. Cancer Res. 2005. 65:11735–11742.28. Herman-Antosiewicz A, Powolny AA, Singh SV. Molecular targets of cancer chemoprevention by garlic-derived organosulfides. Acta Pharmacol Sin. 2007. 28:1355–1364.29. Yuan JP, Wang GH, Ling H, Su Q, Yang YH, Song Y, et al. Diallyl disulfide-induced G2/M arrest of human gastric cancer MGC803 cells involves activation of p38 MAP kinase pathways. World J Gastroenterol. 2004. 10:2731–2734.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of CD40 in Gastric Cancer and Its Effect on the Apoptosis of Gastric Cancer Cells
- Apoptosis Induction of Stomach Cancer Cell by TNF alpha and TGFbeta
- Helicobacter pylori-induced Apoptosis in Gastric Cancer Cell Lines
- Diallyl Disulfide from Garlic Induces Apoptosis through a Caspase Dependent Pathway in Human Breast Cancer Cell Line, MCF-7
- The Activation of Caspase - 3 and Mitogen - activated Protein Kinases during H . Pylori - induced Apoptosis in Gastric Cancer Cells