Korean J Hematol.
2010 Jun;45(2):102-108. 10.5045/kjh.2010.45.2.102.
Fludarabine-based myeloablative regimen as pretransplant conditioning therapy in adult acute leukemia/myelodysplastic syndrome: comparison with oral or intravenous busulfan with cyclophosphamide
- Affiliations
-
- 1Department of Internal Medicine, Dong-A University College of Medicine, Busan, Korea. kshmoon@dau.ac.kr
- 2Clinical Research Center, Dong-A University Hospital, Busan, Korea.
- 3Department of Laboratory Medicine, Dong-A University College of Medicine, Busan, Korea.
- KMID: 2083542
- DOI: http://doi.org/10.5045/kjh.2010.45.2.102
Abstract
- BACKGROUND
A combination of busulfan (Bu) and cyclophosphamide (Cy) has been used as a standard myeloablative regimen for allogeneic hematopoietic stem cell transplantation (HSCT). Recent studies postulate that fludarabine (Flu) is a less toxic substitute for Cy.
METHODS
Forty-two patients who were diagnosed with acute leukemia or myelodysplastic syndrome and received BuFlu (n=17) or BuCy (n=25) from August, 1999 to July, 2009 at Dong-A University Medical Center were retrospectively analyzed.
RESULTS
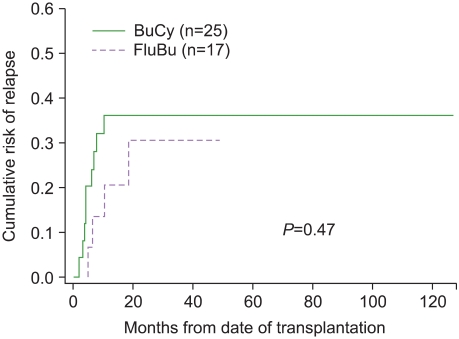
The median follow-up duration was 39.75 months. The BuFlu group showed a lower incidence of mucositis (P=0.005), but there was no significant intergroup difference in the time of engraftment, nausea/vomiting, acute/chronic graft-versus-host disease, hepatic veno-occlusive disease, or hemorrhagic cystitis. Moreover, the 2 groups showed no significant difference in the cumulative risk of relapse, event-free survival, or overall survival.
CONCLUSION
BuFlu administration can be employed as a preparative regimen for allogeneic HSCT and shows efficacy and transplant-adverse effects comparable to those of BuCy. However, randomized prospective studies in more patients are warranted.
Keyword
MeSH Terms
-
Academic Medical Centers
Adult
Behavior Therapy
Busulfan
Cyclophosphamide
Cystitis
Disease-Free Survival
Follow-Up Studies
Graft vs Host Disease
Hematopoietic Stem Cell Transplantation
Hepatic Veno-Occlusive Disease
Humans
Incidence
Leukemia
Mucositis
Myelodysplastic Syndromes
Recurrence
Retrospective Studies
Vidarabine
Busulfan
Cyclophosphamide
Vidarabine
Figure
Reference
-
1. Bhagwatwar HP, Phadungpojna S, Chow DS, Andersson BS. Formulation and stability of busulfan for intravenous administration in high-dose chemotherapy. Cancer Chemother Pharmacol. 1996; 37:401–408. PMID: 8599861.
Article2. Tutschka PJ, Copelan EA, Klein JP. Bone marrow transplantation for leukemia following a new busulfan and cyclophosphamide regimen. Blood. 1987; 70:1382–1388. PMID: 3311203.
Article3. de Lima M, Anagnostopoulos A, Munsell M, et al. Nonablative versus reduced-intensity conditioning regimens in the treatment of acute myeloid leukemia and high-risk myelodysplastic syndrome: dose is relevant for long-term disease control after allogeneic hematopoietic stem cell transplantation. Blood. 2004; 104:865–872. PMID: 15090449.
Article4. Peters WP, Henner WD, Grochow LB, et al. Clinical and pharmacologic effects of high dose single agent busulfan with autologous bone marrow support in the treatment of solid tumors. Cancer Res. 1987; 47:6402–6406. PMID: 2824032.5. McDonald GB, Slattery JT, Bouvier ME, et al. Cyclophosphamide metabolism, liver toxicity, and mortality following hematopoietic stem cell transplantation. Blood. 2003; 101:2043–2048. PMID: 12406916.
Article6. Kashyap A, Wingard J, Cagnoni P, et al. Intravenous versus oral busulfan as part of a busulfan/cyclophosphamide preparative regimen for allogeneic hematopoietic stem cell transplantation: Decreased incidence of hepatic venoocclusive disease (HVOD), HVOD-related mortality, and overall 100-day mortality. Biol Blood Marrow Transplant. 2002; 8:493–500. PMID: 12374454.
Article7. Gandhi V, Plunkett W. Cellular and clinical pharmacology of fludarabine. Clin Pharmacokinet. 2002; 41:93–103. PMID: 11888330.
Article8. Hillmen P. Future prospects for fludarabine-containing regimens in the treatment of hematological cancers. Hematol J. 2004; 5(Suppl 1):S76–S86. PMID: 15079156.
Article9. Giralt S, Estey E, Albitar M, et al. Engraftment of allogeneic hematopoietic progenitor cells with purine analog-containing chemotherapy: harnessing graft-versus-leukemia without myeloablative therapy. Blood. 1997; 89:4531–4536. PMID: 9192777.
Article10. Giralt S, Thall PF, Khouri I, et al. Melphalan and purine analog-containing preparative regimens: reduced-intensity conditioning for patients with hematologic malignancies undergoing allogeneic progenitor cell transplantation. Blood. 2001; 97:631–637. PMID: 11157478.
Article11. Khouri IF, Keating M, Körbling M, et al. Transplant-lite: induction of graft-versus-malignancy using fludarabine-based nonablative chemotherapy and allogeneic blood progenitor-cell transplantation as treatment for lymphoid malignancies. J Clin Oncol. 1998; 16:2817–2824. PMID: 9704734.
Article12. de Lima M, Couriel D, Thall PF, et al. Once-daily intravenous busulfan and fludarabine: clinical and pharmacokinetic results of a myeloablative, reduced-toxicity conditioning regimen for allogeneic stem cell transplantation in AML and MDS. Blood. 2004; 104:857–864. PMID: 15073038.
Article13. Bornhauser M, Storer B, Slattery JT, et al. Conditioning with fludarabine and targeted busulfan for transplantation of allogeneic hematopoietic stem cells. Blood. 2003; 102:820–826. PMID: 12676781.14. Russell JA, Tran HT, Quinlan D, et al. Once-daily intravenous busulfan given with fludarabine as conditioning for allogeneic stem cell transplantation: study of pharmacokinetics and early clinical outcomes. Biol Blood Marrow Transplant. 2002; 8:468–476. PMID: 12374451.
Article15. Chae YS, Sohn SK, Kim JG, et al. New myeloablative conditioning regimen with fludarabine and busulfan for allogeneic stem cell transplantation: comparison with BuCy2. Bone Marrow Transplant. 2007; 40:541–547. PMID: 17637692.
Article16. Andersson BS, de Lima M, Thall PF, et al. Once daily i.v. busulfan and fludarabine (i.v. Bu-Flu) compares favorably with i.v. busulfan and cyclophosphamide (i.v. BuCy2) as pretransplant conditioning therapy in AML/MDS. Biol Blood Marrow Transplant. 2008; 14:672–684. PMID: 18489993.
Article17. Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995; 15:825–828. PMID: 7581076.18. Sullivan KM, Agura E, Anasetti C, et al. Chronic graft-versus-host disease and other late complications of bone marrow transplantation. Semin Hematol. 1991; 28:250–259. PMID: 1887253.19. McDonald GB, Hinds MS, Fisher LD, et al. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Ann Intern Med. 1993; 118:255–267. PMID: 8420443.
Article20. Slattery JT, Kalhorn TF, McDonald GB, et al. Conditioning regimen-dependent disposition of cyclophosphamide and hydroxycyclophosphamide in human marrow transplantation patients. J Clin Oncol. 1996; 14:1484–1494. PMID: 8622062.
Article21. Ciurea SO, Andersson BS. Busulfan in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2009; 15:523–536. PMID: 19361744.
Article22. Schmitz N, Beksac M, Hasenclever D, et al. Transplantation of mobilized peripheral blood cells to HLA-identical siblings with standard-risk leukemia. Blood. 2002; 100:761–767. PMID: 12130483.
Article23. Chunduri S, Dobogai LC, Peace D, et al. Comparable kinetics of myeloablation between fludarabine/full-dose busulfan and fludarabine/ melphalan conditioning regimens in allogeneic peripheral blood stem cell transplantation. Bone Marrow Transplant. 2006; 38:477–482. PMID: 16980995.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Unrelated Bone Marrow Transplantation with a Reduced Toxicity Myeloablative Conditioning Regimen in Wiskott-Aldrich Syndrome
- Allogeneic Stem Cell Transplantation for Patients with Advanced Hematological Malignancies: Comparison of Fludarabine-based Reduced Intensity Conditioning versus Myeloablative Conditioning
- Feasibility of Non-TBI Conditioning with Busulfan and Fludarabine for Allogeneic Stem Cell Transplantation in Lymphoid Malignancy
- Targeted busulfan and fludarabine-based conditioning for bone marrow transplantation in chronic granulomatous disease
- Is Treosulfan-Based Conditioning Attractive as a Reduced-Intensity Conditioning Regimen in Korea?