Korean J Physiol Pharmacol.
2015 Jan;19(1):21-27. 10.4196/kjpp.2015.19.1.21.
Anti-inflammatory, Antioxidant and Antimicrobial Effects of Artemisinin Extracts from Artemisia annua L.
- Affiliations
-
- 1Department of Periodontology, College of Dentistry, Wonkwang University, Iksan 570-749, Korea.
- 2Department of Microbiology, College of Medicine, Chung-Ang University, Seoul 156-756, Korea. kimwy@cau.ac.kr
- 3Department of Pharmacology, College of Pharmacy, Chung-Ang University, Seoul 156-756, Korea.
- 4Asia Pacific International School, Seoul 139-852, Korea.
- 5DOSIS M&M, Seoul 143-891, Korea.
- KMID: 2071815
- DOI: http://doi.org/10.4196/kjpp.2015.19.1.21
Abstract
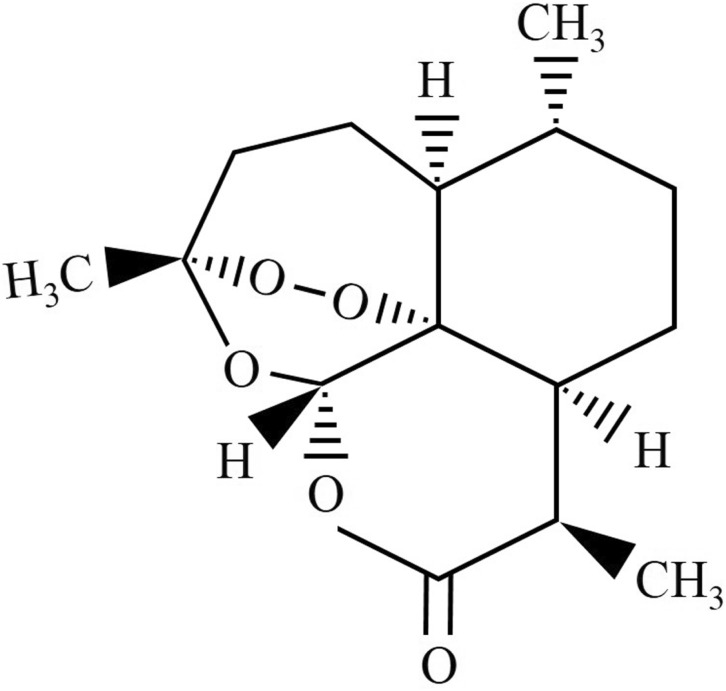
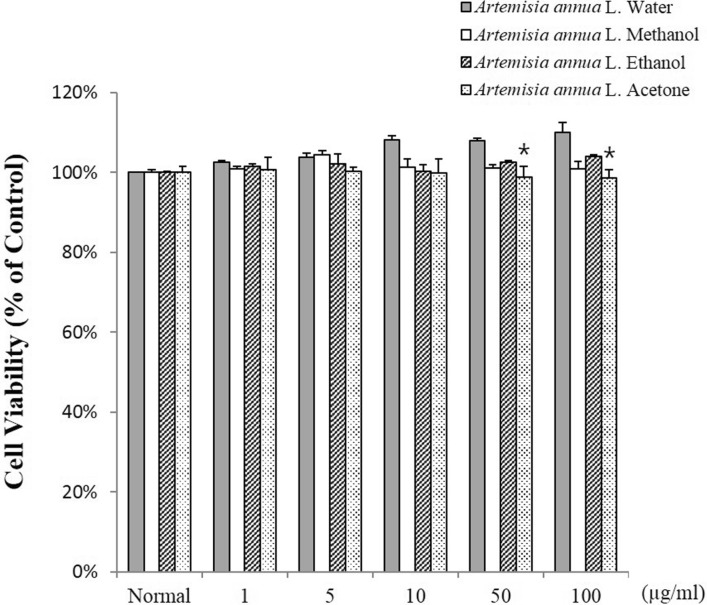
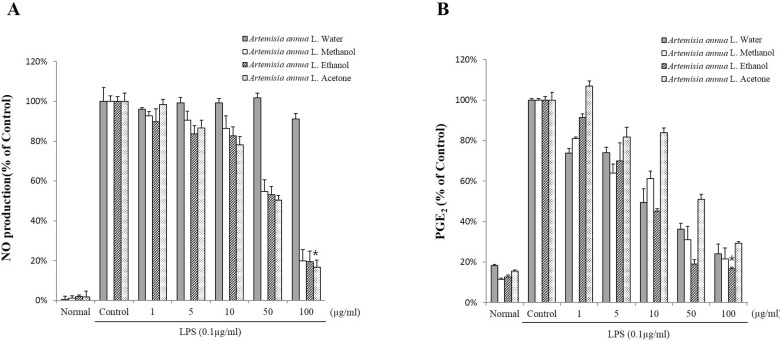
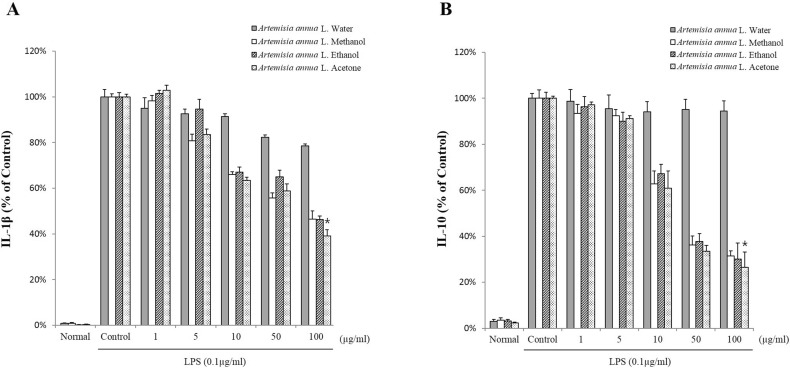
- The anti-inflammatory, antioxidant, and antimicrobial properties of artemisinin derived from water, methanol, ethanol, or acetone extracts of Artemisia annua L. were evaluated. All 4 artemisinin-containing extracts had anti-inflammatory effects. Of these, the acetone extract had the greatest inhibitory effect on lipopolysaccharide-induced nitric oxide (NO), prostaglandin E2 (PGE2), and proinflammatory cytokine (IL-1beta , IL-6, and IL-10) production. Antioxidant activity evaluations revealed that the ethanol extract had the highest free radical scavenging activity, (91.0+/-3.2%), similar to alpha-tocopherol (99.9%). The extracts had antimicrobial activity against the periodontopathic microorganisms Aggregatibacter actinomycetemcomitans, Fusobacterium nucleatum subsp. animalis, Fusobacterium nucleatum subsp. polymorphum, and Prevotella intermedia. This study shows that Artemisia annua L. extracts contain anti-inflammatory, antioxidant, and antimicrobial substances and should be considered for use in pharmaceutical products for the treatment of dental diseases.
MeSH Terms
-
Acetone
Aggregatibacter actinomycetemcomitans
alpha-Tocopherol
Artemisia annua*
Dinoprostone
Ethanol
Fusobacterium nucleatum
Interleukin-6
Methanol
Nitric Oxide
Pharmaceutical Preparations
Prevotella intermedia
Stomatognathic Diseases
Water
Acetone
Dinoprostone
Ethanol
Interleukin-6
Methanol
Nitric Oxide
Pharmaceutical Preparations
Water
alpha-Tocopherol
Figure
Reference
-
1. Taixiang W, Munro AJ, Guanjian L. Chinese medical herbs for chemotherapy side effects in colorectal cancer patients. Cochrane Database Syst Rev. 2005; (1):CD004540. PMID: 15674951.3. Bhakuni RS, Jain DC, Sharma RP, Kumar S. Secondary metabolites of Artemisia annua and their biological activity. Curr Sci India. 2001; 80:35–48.4. de Vries PJ, Dien TK. Clinical pharmacology and therapeutic potential of artemisinin and its derivatives in the treatment of malaria. Drugs. 1996; 52:818–836. PMID: 8957153.
Article5. Efferth T. Willmar Schwabe Award 2006: antiplasmodial and antitumor activity of artemisinin--from bench to bedside. Planta Med. 2007; 73:299–309. PMID: 17354163.6. Konkimalla VB, Blunder M, Korn B, Soomro SA, Jansen H, Chang W, Posner GH, Bauer R, Efferth T. Effect of artemisinins and other endoperoxides on nitric oxide-related signaling pathway in RAW 264.7 mouse macrophage cells. Nitric Oxide. 2008; 19:184–191. PMID: 18472018.7. Ferreira JF, Luthria DL, Sasaki T, Heyerick A. Flavonoids from Artemisia annua L. as antioxidants and their potential synergism with artemisinin against malaria and cancer. Molecules. 2010; 15:3135–3170. PMID: 20657468.8. Lawrence T, Willoughby DA, Gilroy DW. Anti-inflammatory lipid mediators and insights into the resolution of inflammation. Nat Rev Immunol. 2002; 2:787–795. PMID: 12360216.
Article9. Kaplanski G, Marin V, Montero-Julian F, Mantovani A, Farnarier C. IL-6: a regulator of the transition from neutrophil to monocyte recruitment during inflammation. Trends Immunol. 2003; 24:25–29. PMID: 12495721.
Article10. Boscá L, Zeini M, Través PG, Hortelano S. Nitric oxide and cell viability in inflammatory cells: a role for NO in macrophage function and fate. Toxicology. 2005; 208:249–258. PMID: 15691589.
Article11. Ware CF. Network communications: lymphotoxins, LIGHT, and TNF. Annu Rev Immunol. 2005; 23:787–819. PMID: 15771586.
Article12. Halliwell B. Protection against tissue damage in vivo by desferrioxamine: what is its mechanism of action? Free Radic Biol Med. 1989; 7:645–651. PMID: 2695408.
Article13. Morrissey PA, O'Brien NM. Dietary antioxidants in health and disease. Int Dairy J. 1998; 8:463–472.
Article14. Alma MH, Mavi A, Yildirim A, Digrak M, Hirata T. Screening chemical composition and in vitro antioxidant and antimicrobial activities of the essential oils from Origanum syriacum L. growing in Turkey. Biol Pharm Bull. 2003; 26:1725–1729. PMID: 14646179.15. Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005; 366:1809–1820. PMID: 16298220.
Article16. Slots J, Reynolds HS, Genco RJ. Actinobacillus actinomycetemcomitans in human periodontal disease: a cross-sectional microbiological investigation. Infect Immun. 1980; 29:1013–1020. PMID: 6968718.17. Bolstad AI, Jensen HB, Bakken V. Taxonomy, biology, and periodontal aspects of Fusobacterium nucleatum. Clin Microbiol Rev. 1996; 9:55–71. PMID: 8665477.
Article18. Slots J, Ting M. Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in human periodontal disease: occurrence and treatment. Periodontol 2000. 1999; 20:82–121. PMID: 10522224.19. van Steenbergen TJ, Bosch-Tijhof CJ, Petit MD, Van der Velden U. Intra-familial transmission and distribution of Prevotella intermedia and Prevotella nigrescens. J Periodontal Res. 1997; 32:345–350. PMID: 9210087.20. Folin O, Denis W. On phosphotungastic-phosphomo-lybdic compounds as color ragents. J Biol Chem. 1912; 12:239–243.21. Dhingra V, Rajoli C, Narasu ML. Partial purification of proteins involved in the bioconversion of arteannuin B to artemisinin. Bioresource Technol. 2000; 73:279–282.
Article22. Ferrari M, Fornasiero MC, Isetta AM. MTT colorimetric assay for testing macrophage cytotoxic activity in vitro. J Immunol Methods. 1990; 131:165–172. PMID: 2391427.23. Hatano T, Kagawa H, Yasuhara T, Okuda T. Two new flavonoids and other constituents in licorice root: their relative astringency and radical scavenging effects. Chem Pharm Bull (Tokyo). 1988; 36:2090–2097. PMID: 3240445.
Article24. Wikler MA. Clinical and Laboratory Standards Institute. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard. 8th ed. Wayne, Pa: Clinical and Laboratory Standards Institute;2009.25. Scannapieco FA. Periodontal inflammation: from gingivitis to systemic disease? Compend Contin Educ Dent. 2004; 25(7 Suppl 1):16–25. PMID: 15645883.26. Tredwin CJ, Scully C, Bagan-Sebastian JV. Drug-induced disorders of teeth. J Dent Res. 2005; 84:596–602. PMID: 15972585.
Article27. Spencer AJ, Do LG. Changing risk factors for fluorosis among South Australian children. Community Dent Oral Epidemiol. 2008; 36:210–218. PMID: 18474053.
Article28. Ishiguro Y. Mucosal proinflammatory cytokine production correlates with endoscopic activity of ulcerative colitis. J Gastroenterol. 1999; 34:66–74. PMID: 10204613.
Article29. Ponchel F, Morgan AW, Bingham SJ, Quinn M, Buch M, Verburg RJ, Henwood J, Douglas SH, Masurel A, Conaghan P, Gesinde M, Taylor J, Markham AF, Emery P, van Laar JM, Isaacs JD. Dysregulated lymphocyte proliferation and differentiation in patients with rheumatoid arthritis. Blood. 2002; 100:4550–4556. PMID: 12393721.
Article30. Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003; 3:521–533. PMID: 12876555.
Article31. Klotz L, Schmidt M, Giese T, Sastre M, Knolle P, Klockgether T, Heneka MT. Proinflammatory stimulation and pioglitazone treatment regulate peroxisome proliferator-activated receptor gamma levels in peripheral blood mononuclear cells from healthy controls and multiple sclerosis patients. J Immunol. 2005; 175:4948–4955. PMID: 16210596.32. Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006; 83(6 Suppl):1505S–1519S. PMID: 16841861.
Article33. Kawasaki T, Fujimi S, Lederer JA, Hubbard WJ, Choudhry MA, Schwacha MG, Bland KI, Chaudry IH. Trauma-hemorrhage induces depressed splenic dendritic cell functions in mice. J Immunol. 2006; 177:4514–4520. PMID: 16982888.
Article34. Kovatcheva EG, Koleva II, Ilieva M, Pavlov A, Mincheva M, Konushlieva M. Antioxidant activity of extracts from Lavandula vera MM cell cultures. Food Chem. 2001; 72:295–300.35. Ruberto G, Baratta MT, Biondi DM, Amico V. Antioxidant activity of extracts of the marine algal genus Cystoseira in a micellar model system. J Appl Phycol. 2001; 13:403–407.36. Juteau F, Masotti V, Bessière JM, Dherbomez M, Viano J. Antibacterial and antioxidant activities of Artemisia annua essential oil. Fitoterapia. 2002; 73:532–535. PMID: 12385883.37. Cavar S, Maksimovic M, Vidic D, Paric A. Chemical composition and antioxidant and antimicrobial activity of essential oil of Artemisia annua L. from Bosnia. Ind Crop Prod. 2012; 37:479–485.38. Kwamin F, Gref R, Haubek D, Johansson A. Interactions of extracts from selected chewing stick sources with Aggregatibacter actinomycetemcomitans. BMC Res Notes. 2012; 5:203. PMID: 22537711.
Article39. Kanokwiroon K, Teanpaisan R, Wititsuwannakul D, Hooper AB, Wititsuwannakul R. Antimicrobial activity of a protein purified from the latex of Hevea brasiliensis on oral microorganisms. Mycoses. 2008; 51:301–307. PMID: 18924261.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- What is In-Jin-Sook? Artemisia capillaris, Artemisia iwayomogi, and Artemisia annua
- Seizure Induction by Artemisia Annua in an Epilepsy Patient Taking Levetiracetam
- Effect of Artemisia Capillaris Extract on the Growth of Food-Borne Pathogens
- Anti-Obesity Effects of Artemisia annua Extract in Zucker Fatty Rats and High-Fat Diet Sprague Dawley Rats through Upregulation of Uncoupling Protein 1
- Functions of flavonoids in three Korean native varieties of Artemisia species