Korean J Physiol Pharmacol.
2012 Apr;16(2):107-112. 10.4196/kjpp.2012.16.2.107.
3,4,5-Trihydroxycinnamic Acid Inhibits LPS-Induced iNOS Expression by Suppressing NF-kappaB Activation in BV2 Microglial Cells
- Affiliations
-
- 1Department of Pharmacology, College of Medicine, Kangwon National University, Chuncheon 200-701, Korea. wchun@kangwon.ac.kr
- 2College of Pharmacy, Kangwon National University, Chuncheon 200-701, Korea.
- 3Department of Laboratory Animal Resources, National Institute of Food and Drug Evaluation, Korea FDA, Cheongwon 363-951, Korea.
- 4Department of Agricultural Biotechnology, Seoul National University, Seoul 151-742, Korea.
- KMID: 2071755
- DOI: http://doi.org/10.4196/kjpp.2012.16.2.107
Abstract
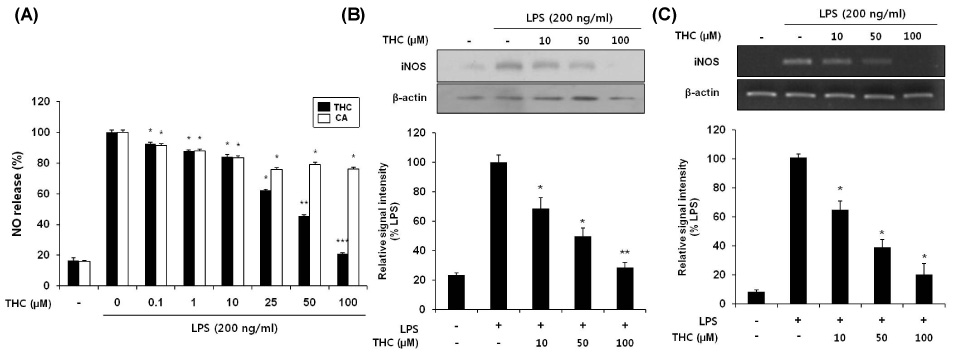
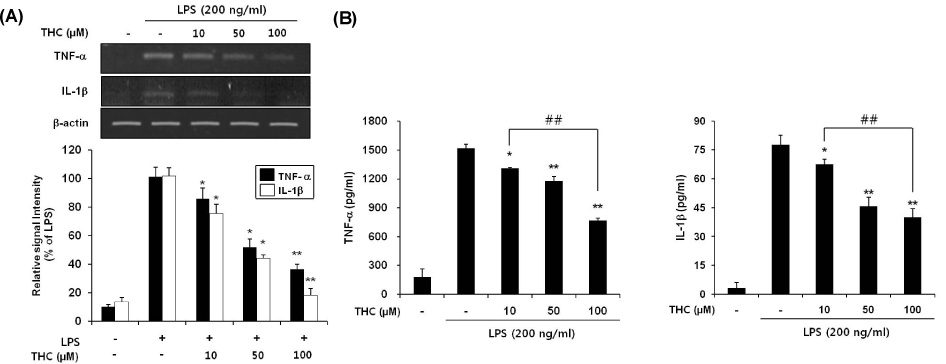
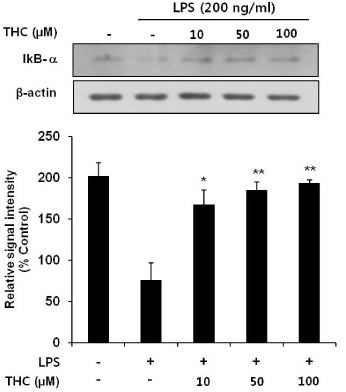
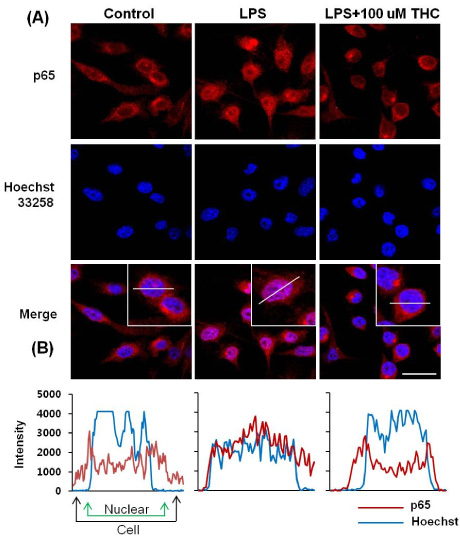
- Although various derivatives of caffeic acid have been reported to possess a wide variety of biological activities such as neuronal protection against excitotoxicity and anti-inflammatory property, the biological activity of 3,4,5-trihydroxycinnamic acid (THC), a derivative of hydroxycinnamic acids, has not been clearly examined. The objective of the present study is to evaluate the anti-inflammatory effects of THC on lipopolysaccharide (LPS)-stimulated BV2 microglial cells. THC significantly suppressed LPS-induced excessive production of nitric oxide (NO) and expression of iNOS, which is responsible for the production of iNOS. THC also suppressed LPS-induced overproduction of pro-inflammatory cytokines such as IL-1beta and TNF-alpha in BV2 microgilal cells. Furthermore, THC significantly suppressed LPS-induced degradation of IkappaB, which retains NF-kappaB in the cytoplasm. Therefore, THC attenuated nuclear translocation of NF-kappaB, a major pro-inflammatory transcription factor. Taken together, the present study for the first time demonstrates that THC exhibits anti-inflammatory activity through the suppression of NF-kappaB transcriptional activation in LPS-stimulated BV2 microglial cells.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Regulatory Effect of 25-hydroxyvitamin D3 on Nitric Oxide Production in Activated Microglia
Jinyoung Hur, Pyeongjae Lee, Mi Jung Kim, Young-Wuk Cho
Korean J Physiol Pharmacol. 2014;18(5):397-402. doi: 10.4196/kjpp.2014.18.5.397.Phosphorylation of Akt Mediates Anti-Inflammatory Activity of 1-
p -Coumaroyl β-D-Glucoside Against Lipopolysaccharide-Induced Inflammation in RAW264.7 Cells
Van Anh Vo, Jae-Won Lee, Ji-Young Kim, Jun-Ho Park, Hee Jae Lee, Sung-Soo Kim, Yong-Soo Kwon, Wanjoo Chun
Korean J Physiol Pharmacol. 2014;18(1):79-86. doi: 10.4196/kjpp.2014.18.1.79.
Reference
-
1. Sugama S. Stress-induced microglial activation may facilitate the progression of neurodegenerative disorders. Med Hypotheses. 2009. 73:1031–1034.2. Perry VH, Gordon S. Macrophages and microglia in the nervous system. Trends Neurosci. 1988. 11:273–277.3. Hailer NP. Immunosuppression after traumatic or ischemic CNS damage: it is neuroprotective and illuminates the role of microglial cells. Prog Neurobiol. 2008. 84:211–233.4. Itagaki S, McGeer PL, Akiyama H, Zhu S, Selkoe D. Relationship of microglia and astrocytes to amyloid deposits of Alzheimer disease. J Neuroimmunol. 1989. 24:173–182.5. Matsumoto H. Some markers reflecting the pathology and disease activity of multiple sclerosis. No To Shinkei. 1992. 44:95–102.6. McGeer PL, McGeer EG. Glial cell reactions in neurodegenerative diseases: pathophysiology and therapeutic interventions. Alzheimer Dis Assoc Disord. 1998. 12:Suppl 2. S1–S6.7. Tuttolomondo A, Di Raimondo D, di Sciacca R, Pinto A, Licata G. Inflammatory cytokines in acute ischemic stroke. Curr Pharm Des. 2008. 14:3574–3589.8. Merrill JE, Benveniste EN. Cytokines in inflammatory brain lesions: helpful and harmful. Trends Neurosci. 1996. 19:331–338.9. Chao CC, Hu S, Close K, Choi CS, Molitor TW, Novick WJ, Peterson PK. Cytokine release from microglia: differential inhibition by pentoxifylline and dexamethasone. J Infect Dis. 1992. 166:847–853.10. Lee IS, Lim J, Gal J, Kang JC, Kim HJ, Kang BY, Choi HJ. Anti-inflammatory activity of xanthohumol involves heme oxygenase-1 induction via NRF2-ARE signaling in microglial BV2 cells. Neurochem Int. 2011. 58:153–160.11. Steinbrecher T, Hrenn A, Dormann KL, Merfort I, Labahn A. Bornyl (3,4,5-trihydroxy)-cinnamate--an optimized human neutrophil elastase inhibitor designed by free energy calculations. Bioorg Med Chem. 2008. 16:2385–2390.12. Nagasaka R, Chotimarkorn C, Shafiqul IM, Hori M, Ozaki H, Ushio H. Anti-inflammatory effects of hydroxycinnamic acid derivatives. Biochem Biophys Res Commun. 2007. 358:615–619.13. Kim YC. Neuroprotective phenolics in medicinal plants. Arch Pharm Res. 2010. 33:1611–1632.14. Lee JW, Cheong IY, Kim HS, Lee JJ, Lee YS, Kwon YS, Kim MJ, Lee HJ, Kim SS, Chun W. Anti-inflammatory activity of 1-docosanoyl cafferate isolated from rhus verniciflua in LPS-stimulated BV2 microglial cells. Korean J Physiol Pharmacol. 2011. 15:9–15.15. Lee Y, Shin DH, Kim JH, Hong S, Choi D, Kim YJ, Kwak MK, Jung Y. Caffeic acid phenethyl ester-mediated Nrf2 activation and IkappaB kinase inhibition are involved in NFkappaB inhibitory effect: structural analysis for NFkappaB inhibition. Eur J Pharmacol. 2010. 643:21–28.16. Rabe C, Steenkamp JA, Joubert E, Burger JF, Ferreira D. Phenolic metabolites from rooibos tea (Aspalathus linearis). Phytochem. 1994. 35:1559–1565.17. Ock J, Kim S, Suk K. Anti-inflammatory effects of a fluorovinyloxyacetamide compound KT-15087 in microglia cells. Pharmacol Res. 2009. 59:414–422.18. Zheng LT, Ryu GM, Kwon BM, Lee WH, Suk K. Anti-inflammatory effects of catechols in lipopolysaccharide-stimulated microglia cells: inhibition of microglial neurotoxicity. Eur J Pharmacol. 2008. 588:106–113.19. Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996. 19:312–318.20. Graeber MB, Streit WJ. Microglia: biology and pathology. Acta Neuropathol. 2010. 119:89–105.21. Aquilano K, Baldelli S, Rotilio G, Ciriolo MR. Role of nitric oxide synthases in Parkinson's disease: a review on the antioxidant and anti-inflammatory activity of polyphenols. Neurochem Res. 2008. 33:2416–2426.22. Ray B, Lahiri DK. Neuroinflammation in Alzheimer's disease: different molecular targets and potential therapeutic agents including curcumin. Curr Opin Pharmacol. 2009. 9:434–444.23. Kuprash DV, Udalova IA, Turetskaya RL, Rice NR, Nedospasov SA. Conserved kappa B element located downstream of the tumor necrosis factor alpha gene: distinct NF-kappa B binding pattern and enhancer activity in LPS activated murine macrophages. Oncogene. 1995. 11:97–106.24. Siebenlist U, Franzoso G, Brown K. Structure, regulation and function of NF-kappa B. Annu Rev Cell Biol. 1994. 10:405–455.25. Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002. 2:725–734.26. Karin M, Takahashi T, Kapahi P, Delhase M, Chen Y, Makris C, Rothwarf D, Baud V, Natoli G, Guido F, Li N. Oxidative stress and gene expression: the AP-1 and NF-kappaB connections. Biofactors. 2001. 15:87–89.27. Moon DO, Park SY, Lee KJ, Heo MS, Kim KC, Kim MO, Lee JD, Choi YH, Kim GY. Bee venom and melittin reduce proinflammatory mediators in lipopolysaccharide-stimulated BV2 microglia. Int Immunopharmacol. 2007. 7:1092–1101.28. Zheng LT, Ock J, Kwon BM, Suk K. Suppressive effects of flavonoid fisetin on lipopolysaccharide-induced microglial activation and neurotoxicity. Int Immunopharmacol. 2008. 8:484–494.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Shikonin Isolated from Lithospermum erythrorhizon Downregulates Proinflammatory Mediators in Lipopolysaccharide-Stimulated BV2 Microglial Cells by Suppressing Crosstalk between Reactive Oxygen Species and NF-kappaB
- Antineuroinflammatory Effects of 7,3’,4’-Trihydroxyisoflavone in Lipopolysaccharide-Stimulated BV2 Microglial Cells through MAPK and NF-κB Signaling Suppression
- 2-Aryl Propionic Acid Amide Modification of Naproxen and Ibuprofen Dimers for Anti-neuroinflammatory Activity in BV2 mouse Microglial Cells
- 3,4,5-Trihydroxycinnamic Acid Inhibits Lipopolysaccharide-Induced Inflammatory Response through the Activation of Nrf2 Pathway in BV2 Microglial Cells
- A new synthetic chalcone derivative, 2-hydroxy-3',5,5'-trimethoxychalcone (DK-139), suppresses the Toll-like receptor 4-mediated inflammatory response through inhibition of the Akt/NF-kappaB pathway in BV2 microglial cells