Korean J Physiol Pharmacol.
2010 Oct;14(5):311-316. 10.4196/kjpp.2010.14.5.311.
P2X and P2Y Receptors Mediate Contraction Induced by Electrical Field Stimulation in Feline Esophageal Smooth Muscle
- Affiliations
-
- 1Department of Pharmacology, College of Pharmacy, Chung-Ang University, Seoul 156-756, Korea. udsohn@cau.ac.kr
- KMID: 2071699
- DOI: http://doi.org/10.4196/kjpp.2010.14.5.311
Abstract
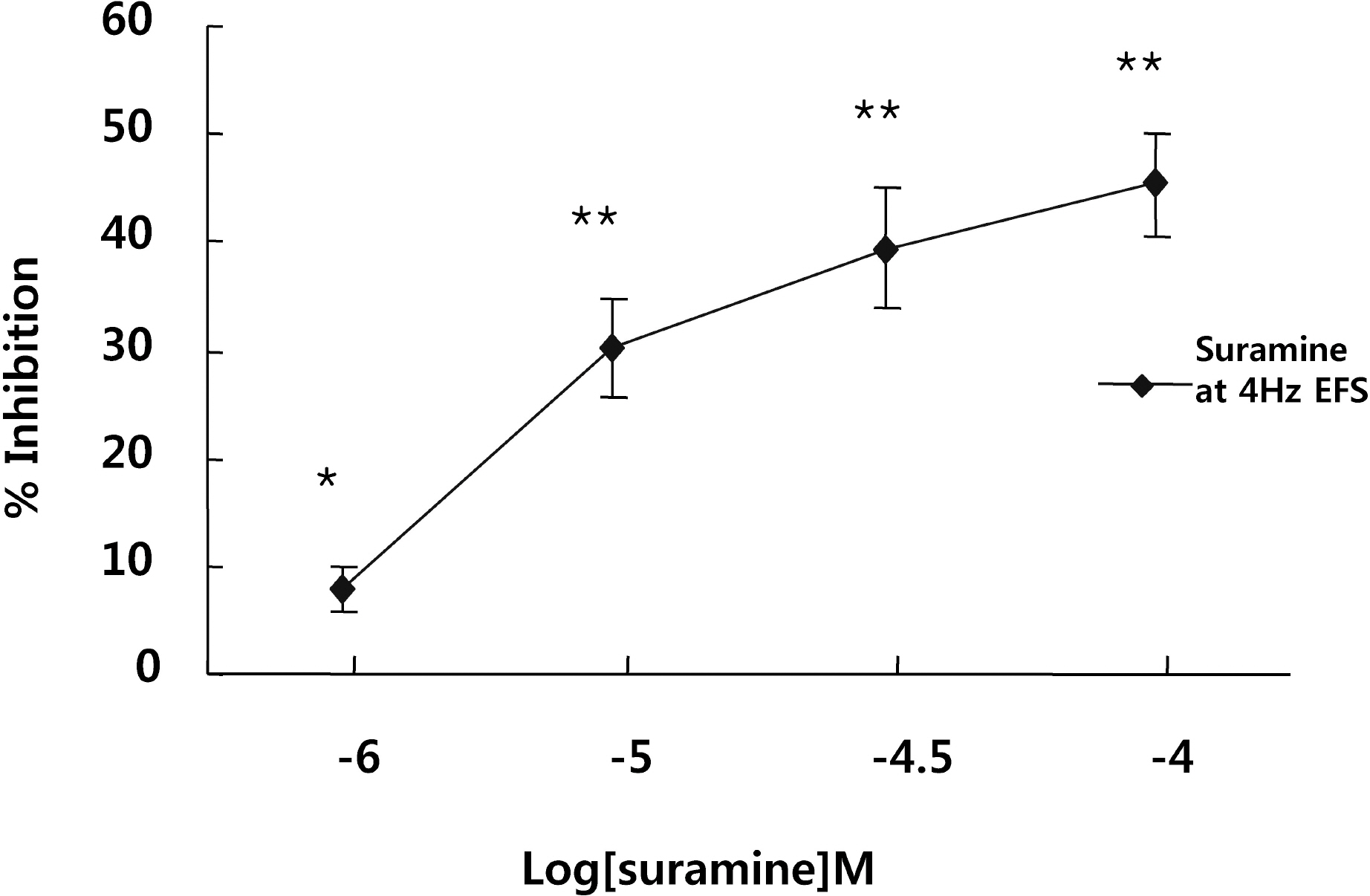
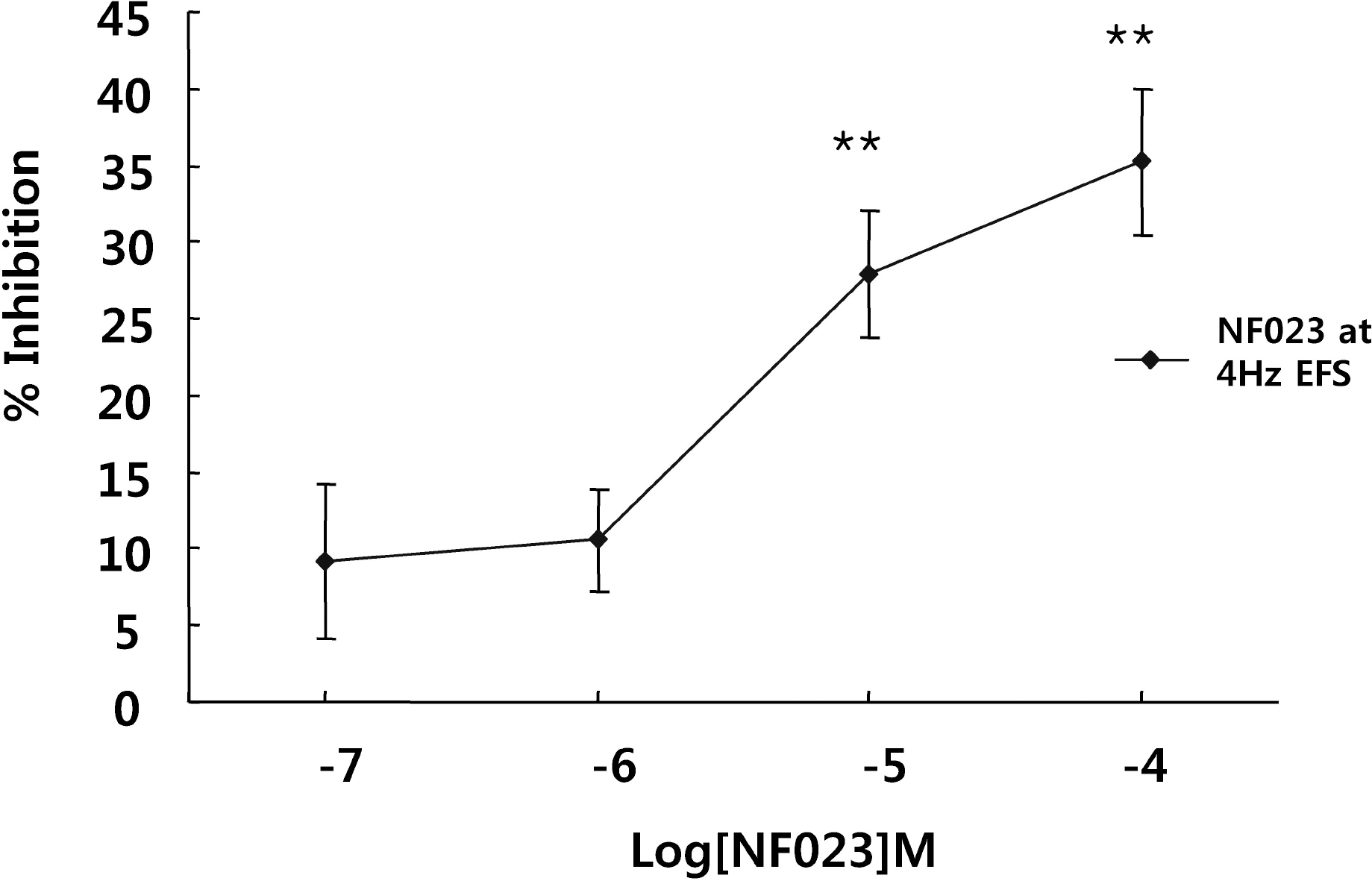
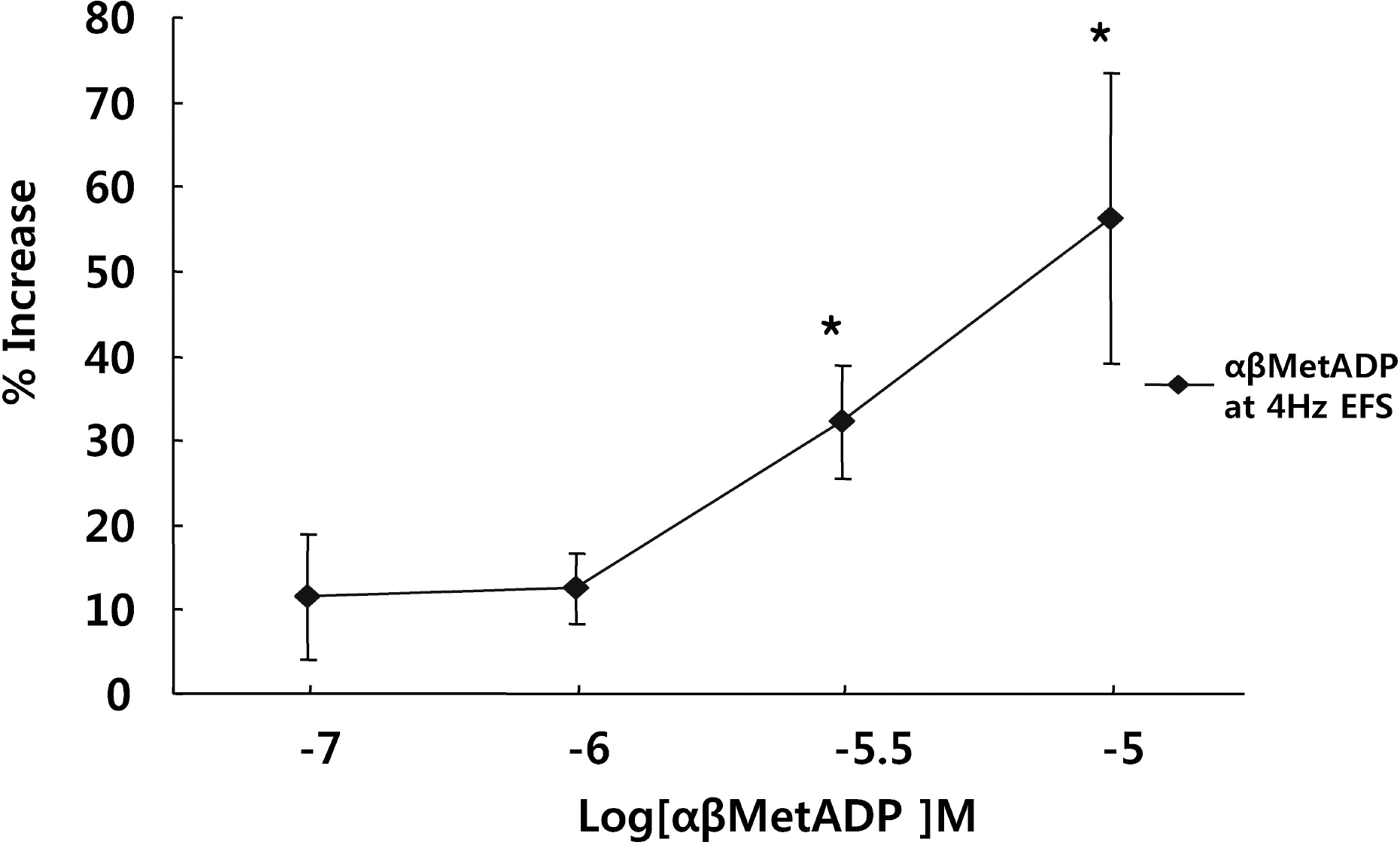
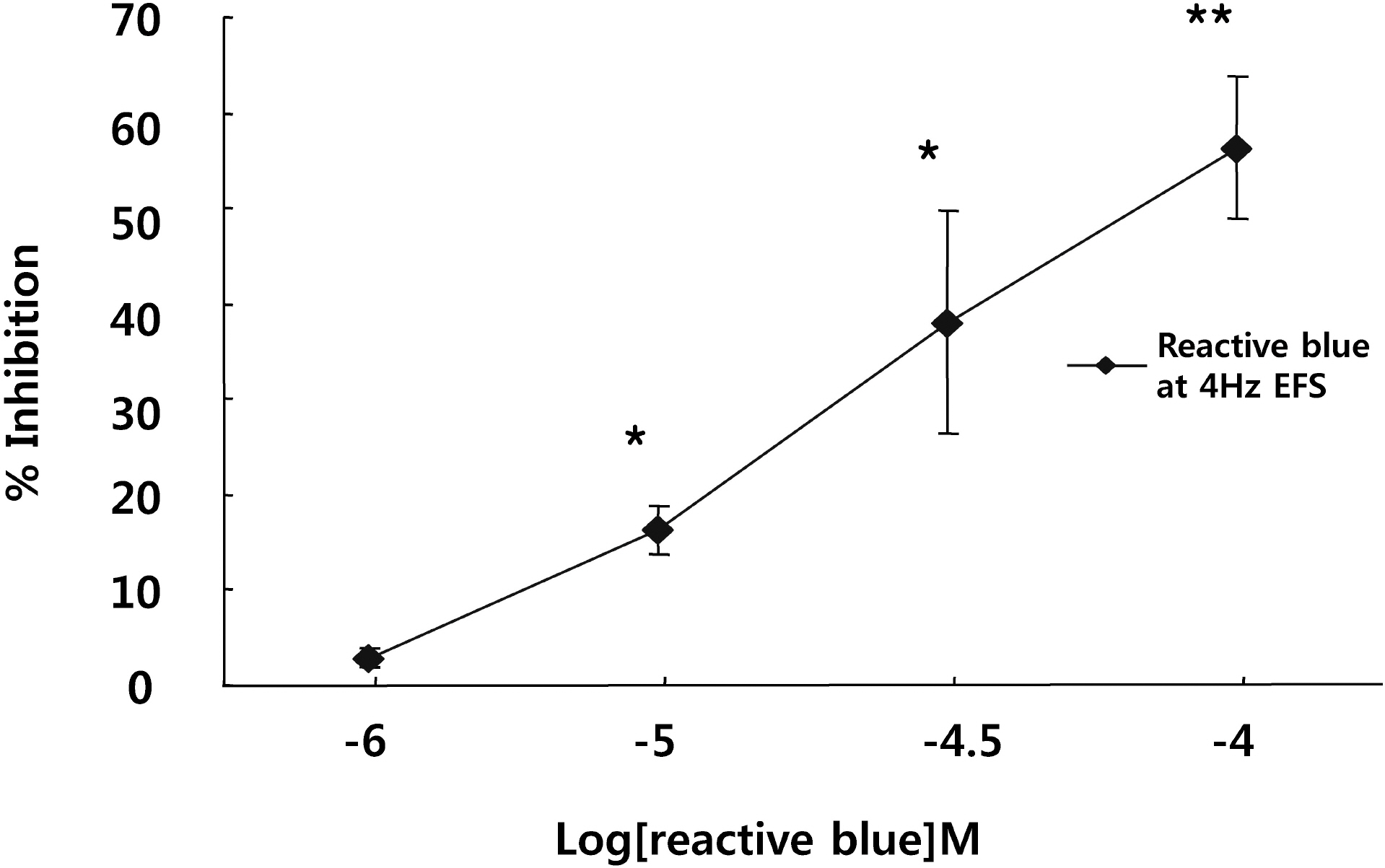
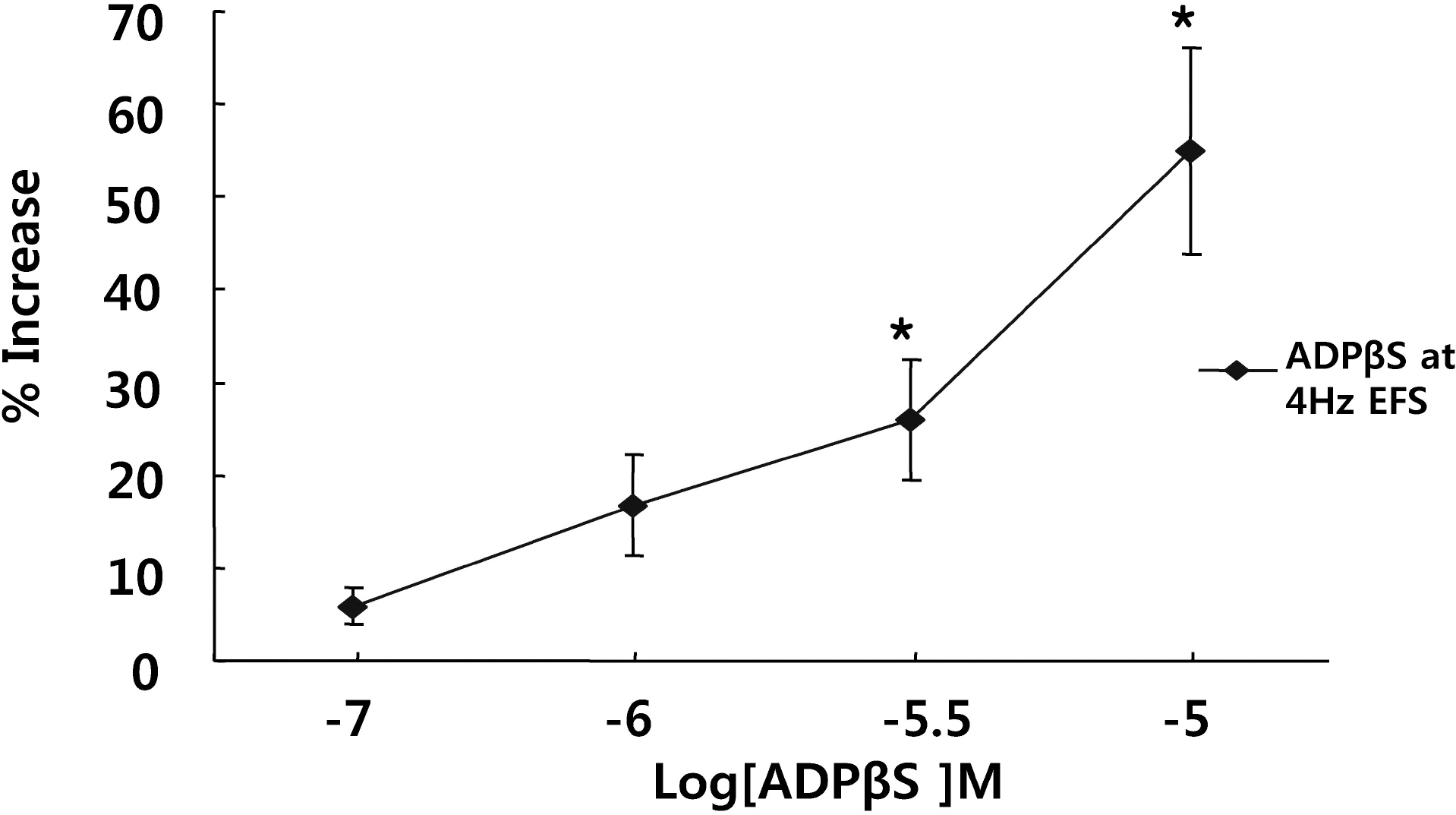
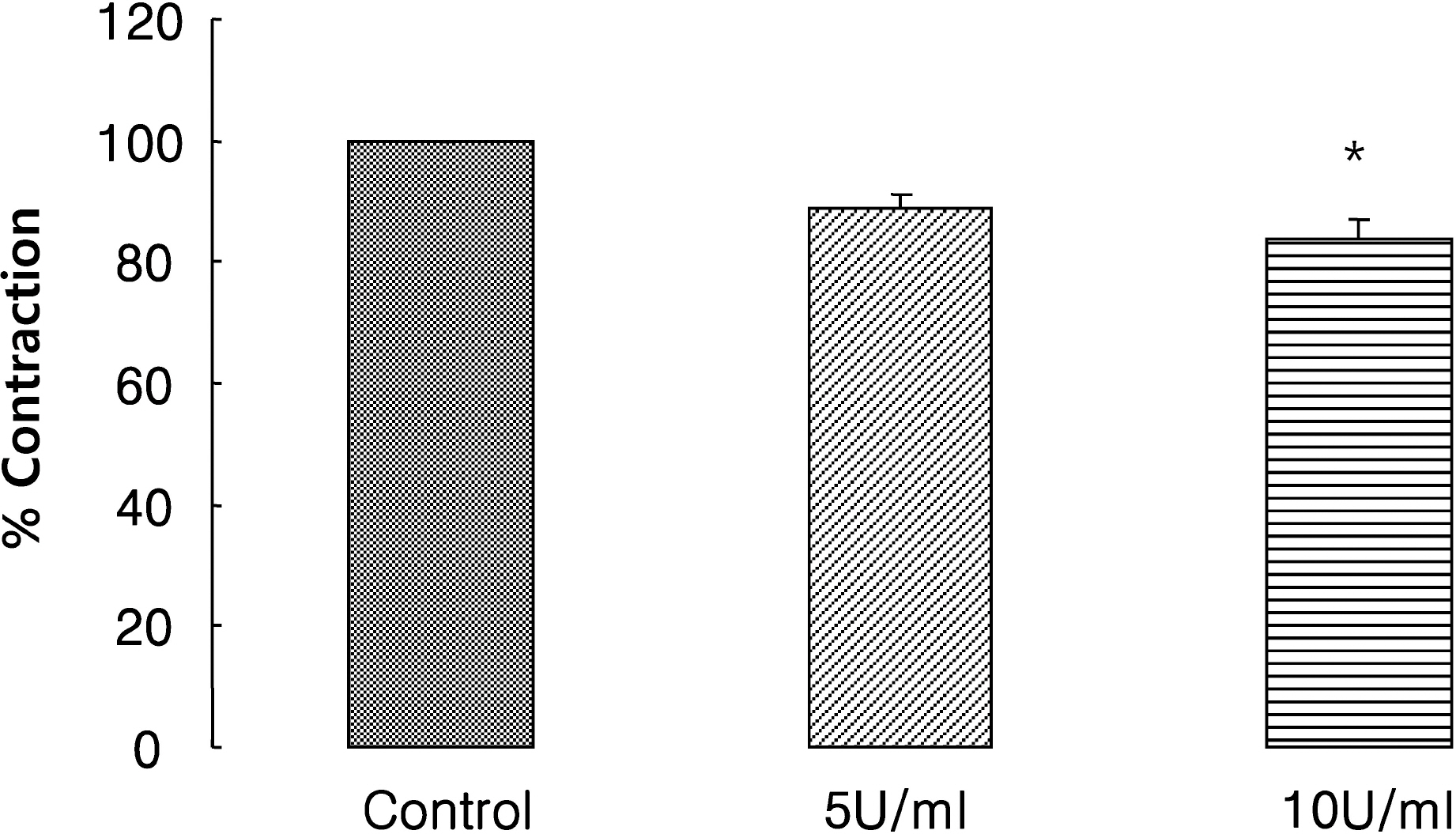
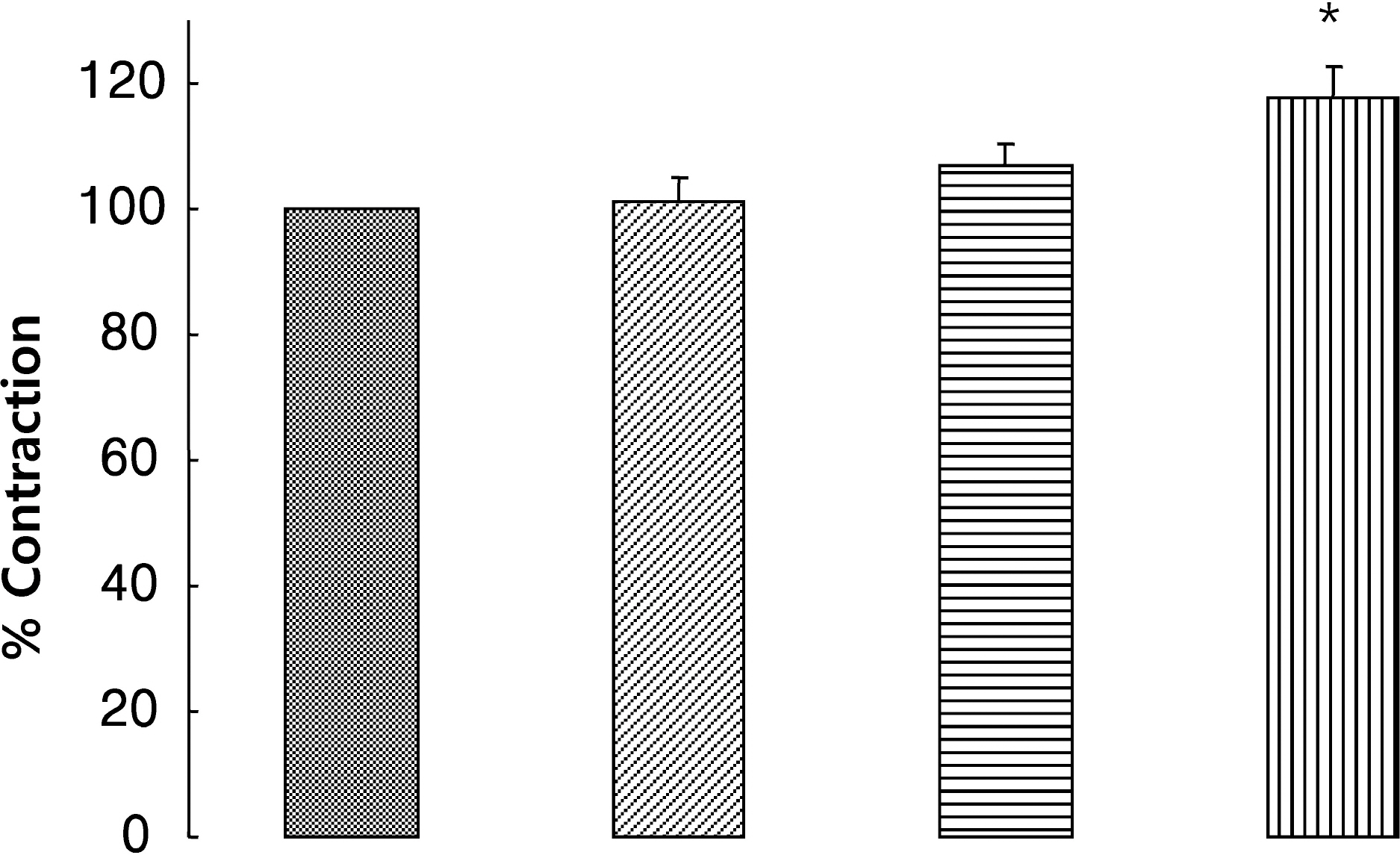
- It is well-known that electrical field stimulation (EFS)-induced contraction is mediated by a cholinergic mechanism and other neurotransmitters. NO, ATP, calcitonin gene-related peptide (CGRP), and substance P are released by EFS. To investigate the purinergic mechanism involved in the EFS-induced contraction, purinegic receptors antagonists were used. Suramine, a non-selective P2 receptor antagonist, reduced the contraction induced by EFS. NF023 (10(-7)~10(-4) M), a selective P2X antagonist, inhibited the contraction evoked by EFS. Reactive blue (10(-6)~10(-4) M), selective P2Y antagonist, also blocked the contraction in a dose-dependent manner. In addition, P2X agonist alpha,beta-methylene 5'-adenosine triphosphate (alphabetaMeATP, 10(-7)~10(-5) M) potentiated EFS-induced contraction in a dose-dependent manner. P2Y agonist adenosine 5'-[beta-thio]diphosphate trilithium salt (ADPbetaS, 10(-7)~10(-5) M) also potentiated EFS-induced contractions in a dose-dependent manner. Ecto-ATPase activator apyrase (5 and 10 U/ml) reduced EFS-induced contractions. Inversely, 6-N,N-diethyl-D-beta,gamma-dibromomethylene 5'-triphosphate triammonium (ARL 67156, 10(-4) M) increased EFS-induced contraction. These data suggest that endogenous ATP plays a role in EFS-induced contractions which are mediated through both P2X-receptors and P2Y-receptors stimulation in cat esophageal smooth muscle.
Keyword
MeSH Terms
-
Adenosine
Adenosine Triphosphatases
Adenosine Triphosphate
Animals
Apyrase
Calcitonin Gene-Related Peptide
Calcium
Cats
Contracts
GTP-Binding Proteins
Muscle, Smooth
Neurotransmitter Agents
Polyphosphates
Substance P
Suramin
Adenosine
Adenosine Triphosphatases
Adenosine Triphosphate
Apyrase
Calcitonin Gene-Related Peptide
Calcium
GTP-Binding Proteins
Neurotransmitter Agents
Polyphosphates
Substance P
Suramin
Figure
Reference
-
References
1. Burnstock G, Campbell G, Satchell D, Smythe A. Evidence that adenosine triphosphate or a related nucleotide is the transmitter substance released by non-adrenergic inhibitory nerves in the gut. Br J Pharmacol. 1970; 40:668–688.
Article2. Lundberg JM. Pharmacology of cotransmission in the autonomic nervous system: Integrative aspects on amines, neuropeptides, adenosine triphosphate, amino acids and nitric oxide. Pharmacol Rev. 1996; 48:113–178.3. Park JH, Kim HS, Park SY, Im C, Jeong JH, Kim IK, Sohn UD. The influences of G proteins, Ca, and K channels on electrical field stimulation in cat esophageal smooth muscle. Korean J Physiol Pharmacol. 2009; 13:393–400.4. Fredholm BB, Abbracchio MP, Burnstock G, Daly JW, Harden TK, Jacobson KA, Leff P, Williams M. Nomenclature and classification of purinoceptors. Pharmacol Rev. 1994; 46:143–156.5. Fredholm BB, Abbracchio MP, Burnstock G, Dubyak GR, Harden TK, Jacobson KA, Schwabe U, Williams M. Towards a revised nomenclature for P1 and P2 receptors. Trends Pharmacol Sci. 1997; 18:79–82.
Article6. Khakh BS, Burnstock G, Kennedy C, King BF, North RA, Seguela P, Voigt M, Humphrey PP. International union of pharmacology. Xxiv. Current status of the nomenclature and properties of P2x receptors and their subunits. Pharmacol Rev. 2001; 53:107–118.7. Vulchanova L, Arvidss on U, Riedl M, Wang J, Buell G, Surprenant A, North RA, Elde R. Differential distribution of two ATP-gated channels (p2x receptors) determined by immunocytochemistry. Proc Natl Acad Sci USA. 1996; 93:8063–8067.
Article8. Collo G, North RA, Kawashima E, Merlo-Pich E, Neidhart S, Surprenant A, Buell G. Cloning of p2×5 and p2×6 receptors and the distribution and properties of an extended family of atp-gated ion channels. J Neurosci. 1996; 16:2495–2507.9. Surprenant A, Buell G, North RA. P2x receptors bring new structure to ligand-gated ion channels. Trends Neurosci. 1995; 18:224–229.
Article10. Werner P, Seward EP, Buell GN, North RA. Domains of p2x receptors involved in desensitization. Proc Natl Acad Sci USA. 1996; 93:15485–15490.
Article11. Bo X, Zhang Y, Nassar M, Burnstock G, Schoepfer R. A P2x purinoceptor cdna conferring a novel pharmacological profile. FEBS Lett. 1995; 375:129–133.
Article12. Buell G, Collo G, Rassendren F. P2x receptors: An emerging channel family. Eur J Neurosci. 1996; 8:2221–2228.
Article13. Schachter JB, Li Q, Boyer JL, Nicholas RA, Harden TK. Second messenger cascade specificity and pharmacological selectivity of the human p2y1-purinoceptor. Br J Pharmacol. 1996; 118:167–173.
Article14. Lustig KD, Shiau AK, Brake AJ, Julius D. Expression cloning of an atp receptor from mouse neuroblastoma cells. Proc Natl Acad Sci USA. 1993; 90:5113–5117.
Article15. Nicholas RA, Watt WC, Lazarowski ER, Li Q, Harden K. Uridine nucleotide selectivity of three phospholipase c-activating p2 receptors: Identification of a UDP-selective, a UTP-selective, and an ATP- and UTP-specific receptor. Mol Pharmacol. 1996; 50:224–229.16. Webb TE, Kaplan MG, Barnard EA. Identification of 6h1 as a p2y purinoceptor: P2y5. Biochem Biophys Res Commun. 1996; 219:105–110.17. Yokomizo T, Izumi T, Chang K, Takuwa Y, Shimizu T. A g-protein-coupled receptor for leukotriene b4 that mediates chemotaxis. Nature. 1997; 387:620–624.
Article18. Xie MS, Dubyak GR. Guanine-nucleotide- and adenine-nucleotide-dependent regulation of phospholipase d in electropermeabilized hl-60 granulocytes. Biochem J. 1991; 278:81–89.
Article19. Zee RY, Michaud SE, Diehl KA, Chasman DI, Emmerich J, Gaussem P, Aiach M, Ridker PM. Purinergic receptor P2y, g-protein coupled, 12 gene variants and risk of incident ischemic stroke, myocardial infarction, and venous thromboembolism. Atherosclerosis. 2008; 197:694–699.
Article20. Lazarowski ER, Boucher RC, Harden TK. Calcium-dependent release of arachidonic acid in response to purinergic receptor activation in airway epithelium. Am J Physiol. 1994; 266:C406–415.
Article21. Dubyak GR, el-Moatassim C. Signal transduction via p2-purinergic receptors for extracellular atp and other nucleotides. Am J Physiol. 1993; 265:C577–606.
Article22. Harden TK, Boyer JL, Nicholas RA. P2-purinergic receptors: Subtype-associated signaling responses and structure. Annu Rev Pharmacol Toxicol. 1995; 35:541–579.
Article23. Nagaoka M, Nara M, Tamada T, Kume H, Oguma T, Kikuchi T, Zaini J, Moriya T, Ichinose M, Tamura G, Hattori T. Regulation of adenosine 5′-triphosphate (atp)-gated p2x(4) receptors on tracheal smooth muscle cells. Respir Physiol Neurobiol. 2009; 166:61–67.
Article24. Li RW, Seto SW, Au AL, Kwan YW, Chan SW, Lee SM, Tse CM, Leung GP. Inhibitory effect of nonsteroidal anti-inflammatory drugs on adenosine transport in vascular smooth muscle cells. Eur J Pharmacol. 2009; 612:15–20.
Article25. Kadowaki M, Takeda M, Tokita K, Hanaoka K, Tomoi M. Molecular identification and pharmacological characterization of adenosine receptors in the guinea-pig colon. Br J Pharmacol. 2000; 129:871–876.
Article26. Moneta NA, McDonald TJ, Cook MA. Endogenous adenosine inhibits evoked substance P release from perifused networks of myenteric ganglia. Am J Physiol. 1997; 272:G38–45.
Article27. Burnstock G, Knight GE. Cellular distribution and functions of p2 receptor subtypes in different systems. Int Rev Cytol. 2004; 240:31–304.
Article28. Preiksaitis HG, Krysiak PS, Chrones T, Rajgopal V, Laurier LG. Pharmacological and molecular characterization of muscarinic receptor subtypes in human esophageal smooth muscle. J Pharmacol Exp Ther. 2000; 295:879–888.29. Wang J, Krysiak PS, Laurier LG, Sims SM, Preiksaitis HG. Human esophageal smooth muscle cells express muscarinic receptor subtypes m(1) through m(5). Am J Physiol Gastrointest Liver Physiol. 2000; 279:G1059–1069.
Article30. Park SY, Shim JH, Kim M, Sun YH, Kwak HS, Yan X, Choi BC, Im C, Sim SS, Jeong JH, Kim IK, Min YS, Sohn UD. Mlck and PKC involvements via gi and rho a protein in contraction by the electrical field stimulation in feline esophageal smooth muscle. Korean J Physiol Pharmacol. 2010; 14:29–35.
Article31. Heppner TJ, Werner ME, Nausch B, Vial C, Evans RJ, Nelson MT. Nerve-evoked purinergic signalling suppresses action potentials, Ca2+ flashes and contractility evoked by muscarinic receptor activation in mouse urinary bladder smooth muscle. J Physiol. 2009; 587:5275–5288.32. Sohn UD, Harnett KM, De Petris G, Behar J, Biancani P. Distinct muscarinic receptors, G proteins and phospholipases in esophageal and lower esophageal sphincter circular muscle. J Pharmacol Exp Ther. 1993; 267:1205–1214.33. Giaroni C, Knight GE, Ruan HZ, Glass R, Bardini M, Lecchini S, Frigo G, Burnstock G. P2 receptors in the murine gastrointestinal tract. Neuropharmacology. 2002; 43:1313–1323.
Article34. Murthy KS, Makhlouf GM. Coexpression of ligand-gated p2x and g protein-coupled p2y receptors in smooth muscle. Preferential activation of p2y receptors coupled to phospholipase c (plc)-beta1 via galphaq/11 and to plc-beta3 via gbetagammai3. J Biol Chem. 1998; 273:4695–4704.35. Jin Z, Guo HS, Xu DY, Hong MY, Li XL, Xu WX. Effects of purinergic analogues on spontaneous contraction and electrical activities of gastric antral circular muscle in guinea-pig. Sheng Li Xue Bao. 2004; 56:678–684.36. Bultmann R, Wittenburg H, Pause B, Kurz G, Nickel P, Starke K. P2-purinoceptor antagonists: Iii. Blockade of P2-purinoceptor subtypes and ecto-nucleotidases by compounds related to suramin. Naunyn Schmiedebergs Arch Pharmacol. 1996; 354:498–504.37. Bean BP. Pharmacology and electrophysiology of ATP-activated ion channels. Trends Pharmacol Sci. 1992; 13:87–90.
Article38. Bogdanov Y, Rubino A, Burnstock G. Characterisation of subtypes of the P2x and P2y families of ATP receptors in the foetal human heart. Life Sci. 1998; 62:697–703.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Enflurane on the Tracheal Smooth Muscle Contracted with Electrical Field Stimulation in the Guinea Pig
- Evidence for adenosine triphosphate (ATP) as an excitatory neurotransmitter in guinea-pig gastric antrum
- Characteristics of 5-Hydroxytryptamine Receptors Involved in Contraction of Feline Ileal Longitudinal Smooth Muscle
- MLCK and PKC Involvements via Gi and Rho A Protein in Contraction by the Electrical Field Stimulation in Feline Esophageal Smooth Muscle
- Effects of Several Autonomic Drugs on the Responses of the Isolated Rabbit Detrusor Muscle Strips to Electrical Stimulation