Korean J Physiol Pharmacol.
2009 Jun;13(3):215-220. 10.4196/kjpp.2009.13.3.215.
Block of hERG K+ Channel by Classic Histamine H1 Receptor Antagonist Chlorpheniramine
- Affiliations
-
- 1Department of Physiology, Institute of Bioscience and Biotechnology, Kangwon National University College of Medicine, Chuncheon 200-701, Korea. suhyunjo@kangwon.ac.kr
- KMID: 2071674
- DOI: http://doi.org/10.4196/kjpp.2009.13.3.215
Abstract
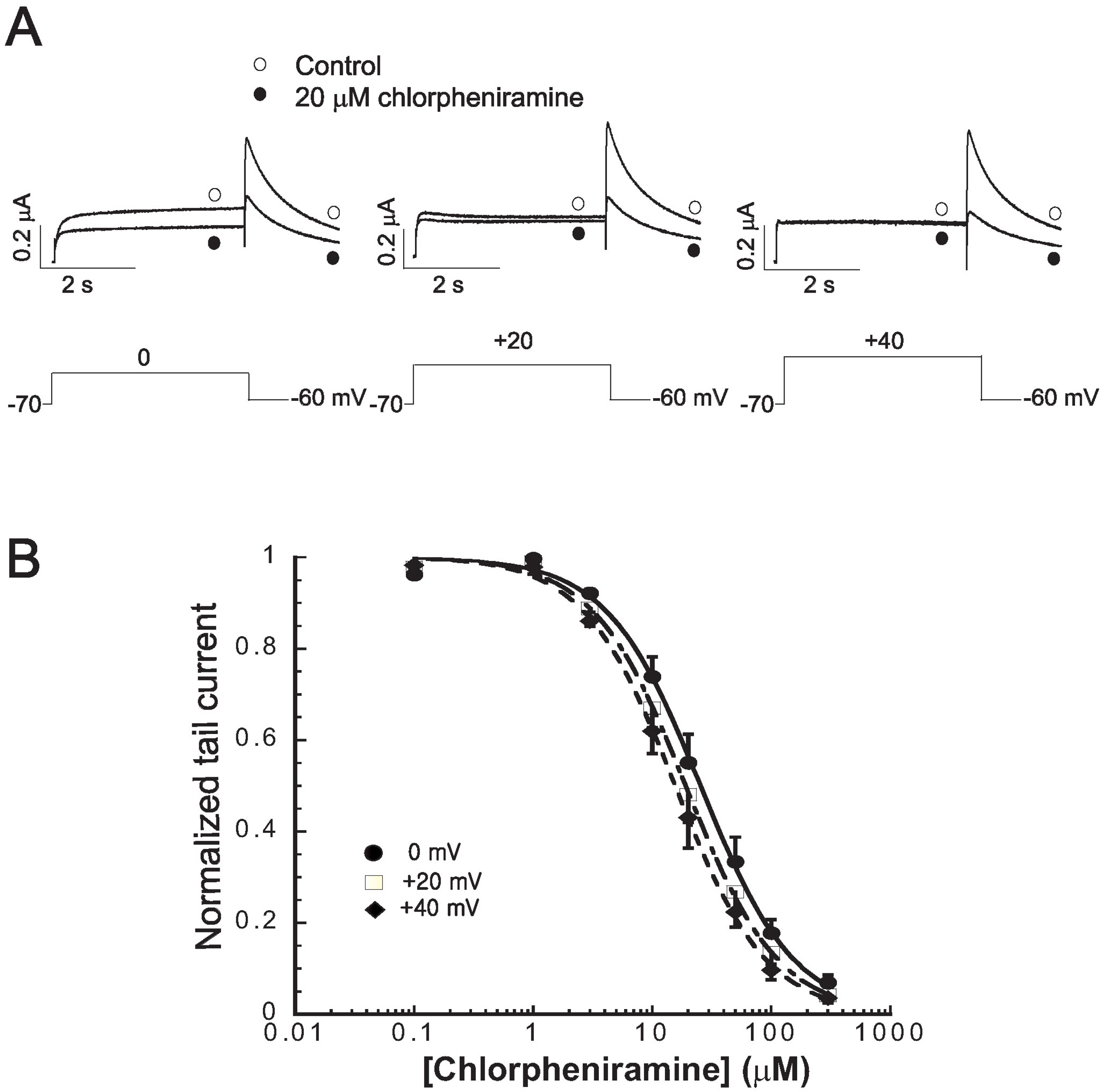
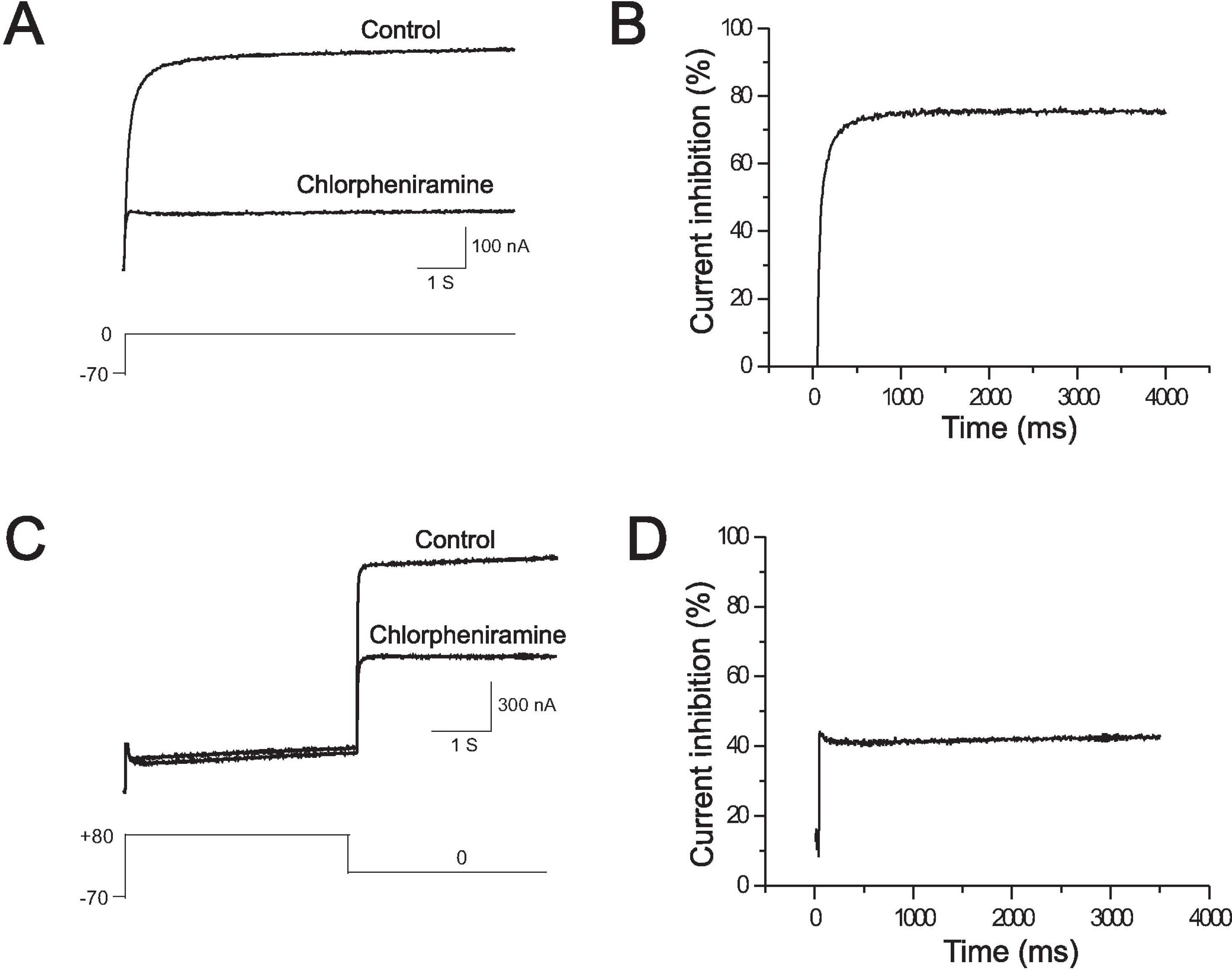
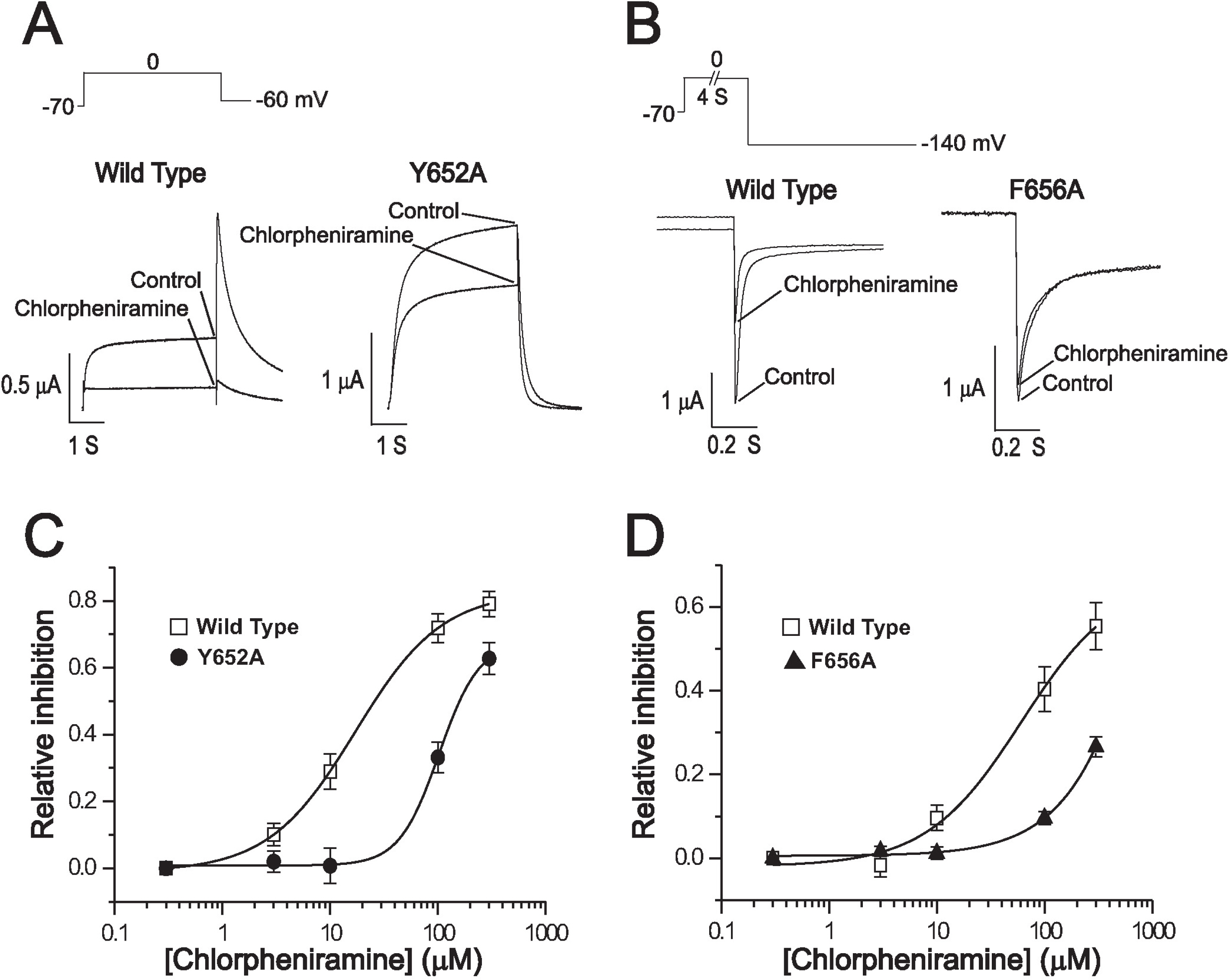
- Chlorpheniramine is a potent first-generation histamine H1 receptor antagonist that can increase action potential duration and induce QT prolongation in several animal models. Since block of cardiac human ether-a-go-go-related gene (hERG) channels is one of leading causes of acquired long QT syndrome, we investigated the acute effects of chlorpheniramine on hERG channels to determine the electrophysiological basis for its proarrhythmic potential. We examined the effects of chlorpheniramine on the hERG channels expressed in Xenopus oocytes using two-microelectrode voltage-clamp techniques. Chlorpheniramine induced a concentration-dependent decrease of the current amplitude at the end of the voltage steps and hERG tail currents. The IC50 of chlorpheniramine-dependent hERG block in Xenopus oocytes decreased progressively relative to the degree of depolarization. Chlorpheniramine affected the channels in the activated and inactivated states but not in the closed states. The S6 domain mutations Y652A and F656A partially attenuated (Y652A) or abolished (F656A) the hERG current block. These results suggest that the H1 antihistamine, chlorpheniramine is a blocker of the hERG channels, providing a molecular mechanism for the drug-induced arrhythmogenic side effects.
Keyword
MeSH Terms
Figure
Reference
-
Arcangeli A., Becchetti A., Mannini A., Mugnai G., De Pilippi P., Tarone G., Del Bene MR., Barletta E., Wanke E., Olivotto M. Integrin-mediated neurite outgrowth in neuroblastoma cells depends on the activation of potassium channels. J Cell Biol. 122:1131–1143. 1993.
ArticleArcangeli A., BIanchi L., Becchetti A., Faravelli L., Coronnello M., Mini E., Olivotto M., Wanke E. A novel inward-rectifier K+ current with a cell-cycle dependence governs the resting potential of mammalian neuroblastoma cells. J Physiol (Lond). 489:455–471. 1995.Baselt R., Cravey R. Disposition of toxic drugs and chemicals in man. 4th ed.Foster City, CA: Chemical Toxicology Institute;1995.Chiesa N., Rosati B., Arcangeli A., Olivotto M., Wanke E. A novel role for HERG K+ channels: spike-frequency adaptation. J Physiol (Lond). 501:313–318. 1997.Choi SY., Koh YS., Jo SH. Inhibition of human ether-a-go-go-related gene K+ channel and IKr of guinea pig cardiomyocytes by antipsychotic drug trifluoperazine. J Pharmacol Exp Ther. 313:888–895. 2005.Craft TM. Torsade de pointes after astemizole overdose. Br Med J. 292:60. 1986.
ArticleGoldberg MJ., Spector R., Chiang CK. Transport of diphenhydramine in the central nervous system. J Pharmacol Exp Ther. 240:717–722. 1987.Hestand HE., Teske DW. Diphenhydramine hydrochloride intoxication. J Pediatr. 90:1017–1018. 1977.
ArticleHuang SM., Chiou WL. Pharmacokinetics and tissue distribution of chlorpheniramine in rabbits after intravenous administration. J Pharmacokinet Biopharm. 9:711–723. 1981.
ArticleHung YM., Chang JC. Weight-reducing regimen associated with polymorphic ventricular tachycardia. Am J Emerg Med. 24:714–716. 2006.
ArticleKatzung BG. Histamine, Serotonin, and the ergot alkaloids. Basic and Clinical Pharmacology. 9th ed.McGraw-Hill;San Francisco: p. p. 263–268. 2003.Ki I., Inui A., Ito T. Effects of histamine H1 receptor antagonists on action potentials in guinea-pig isolated papillary muscles. Arch Int Pharmacodyn Ther. 331:59–73. 1996.Mitcheson JS. Drug binding to HERG channels: evidence for a “non-aromatic” binding site for fluvoxamine. Br J Pharmacol. 139:883–884. 2003.
ArticleMitcheson JS., Chen J., Lin M., Culberson C., Sanguinetti MC. A structural basis for drug-induced long QT syndrome. Proc Natl Acad Sci USA. 97:12329–12333. 2000.
ArticleRedfern WS., Carlsson L., Davis AS., Lynch WG., MacKenzie I., Palethorpe S., Siegl PK., Strang I., Sullivan AT., Wallis R., Camm AJ., Hammond TG. Relationships between preclinical cardiac electrophysiology, clinical QT interval prolongation and torsade de pointes for a broad range of drugs: evidence for a provisional safety margin in drug development. Cardiovasc Res. 58:32–45. 2003.
ArticleReed D. A fatal case involving chlorpheniramine. Clin Toxicol. 18:941–943. 1981.
ArticleSànchez- Chapula JA., Navarro-Polanco RA., Culberson C., Chen J., Sanguinetti MC. Molecular determinants of voltage-dependent human ether-a-go-go related gene (HERG) K+ channel block. J Biol Chem. 277:23587–23595. 2002.Suessbrich H., Waldegger S., Lang F., Busch AE. Blockade of HERG channels expressed in Xenopus oocytes by the histamine receptor antagonists terfenadine and astemizole. FEBS Lett. 385:77–80. 1996.
ArticleTagawa M., Kano M., Okamura N., Higuchi M., Matsuda M., Mizuki Y., Arai H., Fujii T., Komemushi S., Itoh M., Sasaki H., Watanabe T., Yanai K. Differential cognitive effects of ebastine and (+)-chlorpheniramine in healthy subjects: correlation between cognitive impairment and plasma drug concentration. Br J Clin Pharmacol. 53:296–304. 2002.
ArticleTagawa M., Kano M., Okamura N., Higuchi M., Matsuda M., Mizuki Y., Arai H., Iwata R., Fujii T., Komemushi S., Ido T., Itoh M., Sasaki H., Watanabe T., Yanai K. Neuroimaging of histamine H1-receptor occupancy in human brain by positron emission tomography (PET): a comparative study of ebastine, a second-generation antihistamine, and (+)-chlorpheniramine, a classical antihistamine. Br J Clin Pharmacol. 52:501–509. 2001.
ArticleTaglialatela M., Castaldo P., Pannaccione A., Giorgio G., Genovese A., Marone G., Annunziato L. Cardiac ion channels and antihistamines: possible mechanisms of cardiotoxicity. Clin Exp Allergy. 29:182–189. 1999.
ArticleTaglialatela M., Timmerman H., Annunziato L. Cardiotoxic potential and CNS effects of first-generation antihistamines. Trends Pharmacol Sci. 21:52–56. 2000.
ArticleTie H., Walker BD., Valenzuela SM., Breit SN., Campbell TJ. The heart of psychotropic drug therapy. Lancet. 355:1825. 2000.
ArticleVenkatraman N., O'Neil D., Hall AP. Life-threatening overdose with lamotrigine, citalopram, and chlorpheniramine. J Postgrad Med. 54:316–317. 2008.
ArticleWymore RS., Gintant GA., Wymore RT., Dixon JE., Mckinnon D., Cohen IS. Tissue and species distribution of mRNA for the IKr-like K+ channel, erg. Circ Res. 80:261–268. 1996.Zhou Z., Gong Q., Ye B., Fan Z., Makielski JC., Robertson GA., January CT. Properties of HERG channels stably expressed in HEK 293 cells studied at physiological temperature. Biophys J. 74:230–241. 1998.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of H1 and H2 Antihistaminics on Dermographism
- Anaphylaxis to Chlorpheniramine Maleate and Literature Review
- The role of H1- and H2-receptors in the effect of compound 48/80 in the asphyxiation and body temperature of mice
- The Effect of H1 and H2 Blockade on Cutaneous Histamine Response in Man
- Effects of Histamine 2 Antagonist in the Treatment of Perennial Allergic Rhinitis