J Bacteriol Virol.
2006 Sep;36(3):133-139. 10.4167/jbv.2006.36.3.133.
Antimicrobial Resistance and Integrons Found in Commensal Escherichia coli Isolates from Healthy Humans
- Affiliations
-
- 1Department of Microbiology, Kyungpook National University School of Medicine, Daegu, Korea. yclee@knu.ac.kr
- KMID: 2055020
- DOI: http://doi.org/10.4167/jbv.2006.36.3.133
Abstract
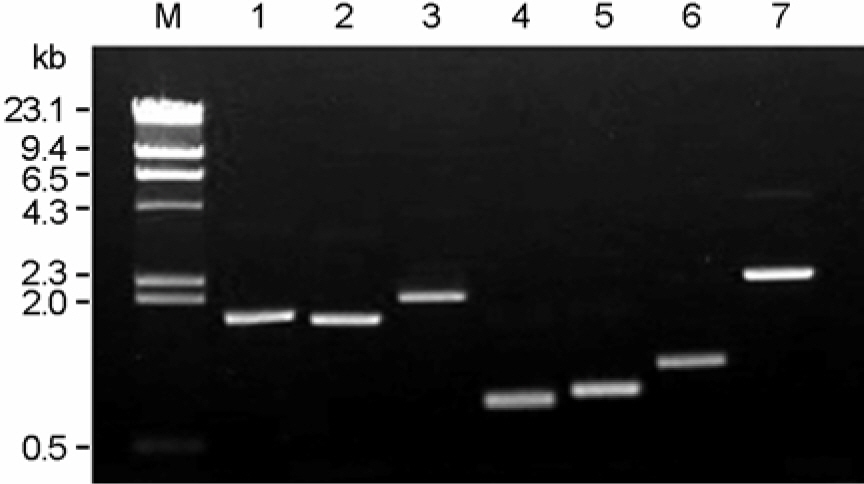
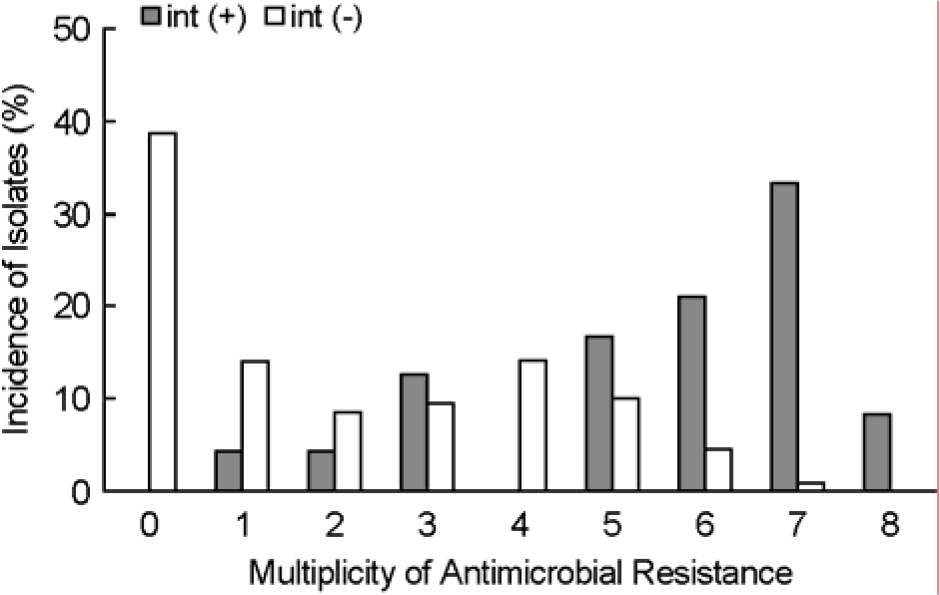
- The emergence and spread of antimicrobial resistance among the pathogenic and commensal Enterobacteriaceae are of great concern worldwide. We characterized the antimicrobial resistance and integrons found in commensal Escherichia coli from healthy humans in the community. Class 1 integrase (intl1) and class 2 integrase (intl2) genes were identified in 22 (13.3%) and 2 (1.2%) of 165 E. coli isolates, respectively. dfrA17-aadA5 and dfrA1-aadA2 were the most common class 1 integrons. The prevalence of each type of class 1 integron among commensal E. coli isolates during 2001~2003 was similar to that of clinical E. coli isolates from hospital-acquired infections during 1994~1999. The resistant rates of commensal E. coli isolates carrying intl1 to ampicillin, streptomycin, gentamicin, sulfamethoxazole, trimethoprim, chloramphenicol, and tetracycline were significantly higher than those of intl1-negative E. coli isolates (p<0.05). Integrons were directly associated with multidrug resistance in commensal E. coli isolates. It is hypothesized that multidrug-resistant Enterobacteriaceae from hospital-acquired infections are a potential reservoir for integrons associated with resistance genes found in commensal E. coli isolates in the community
MeSH Terms
Figure
Reference
-
References
1). Birnboim IC, Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 7:1513–1523. 1979.
Article2). Collis CM, Grammaticopoulos G, Briton J, Stockes HW, Hall RM. Site-specific insertion of gene cassettes into integrons. Mol Microbiol. 9:41–52. 1993.
Article3). Fluit AC, Schmitz FJ. Class 1 integrons, gene cassettes, mobility, and epidemiology. Eur J Clin Microbiol Infect Dis. 18:761–770. 1999.
Article4). Goldstein C, Lee MD, Sanchez S, Hudson C, Phillips B, Register B, Grady M, Liebert C, Summers AO, White DG, Maurer JJ. Incidence of class 1 and 2 integrase in clinical and commensal bacteria from livestock, companion, animals, and exotics. Antimicrob Agents Chemother. 45:723–726. 2001.5). Guerra B, Junker E, Schroeter A, Malorny B, Lehmann S, Helmuth R. Phenotypic and genotypic characterization of antimicrobial resistance in German Escherichia coli isolates from cattle, swine and poultry. J Antimicrob Chemother. 52:489–492. 2003.6). Hall RM, Collis CM. Mobile gene cassettes and integrons: capture and spread of genes by site-specific recombination. Mol Microbiol. 15:593–600. 1995.
Article7). Hall RM, Collis CM. Antibiotic resistance in gram-negative bacteria: the role of gene cassettes and integrons. Drug Resist. Updates. 1:109–119. 1998.8). Kang HY, Jeong YS, Oh JY, Tae SH, Choi CH, Moon DC, Lee WK, Lee YC, Seol SY, Cho DT, Lee JC. Characterization of antimicrobial resistance and class 1 integrons found in Escherichia coli isolates from humans and animals in Korea. J Antimicrob Chemother. 55:639–644. 2005.9). Kim KS, Oh JY, Jeong YW, Cho JW, Park JC, Cho DT, Lee JC. Epidemiological typing and characterization of dfr genes of Shigella sonnei isolates in Korea during the last two decades. J Microbiol Biotechnol. 12:106–113. 2002.10). Lee JC, Oh JY, Cho JW, Park JC, Kim JM, Seol SY, Cho DT. The prevalence of trimethoprim-resistance-conferring dihydrofolate reductase genes in urinary isolates of Escherichia coli in Korea. J Antimicrob Chemother. 47:599–604. 2001.11). Leverstein-van Hall MA, Box ATA, Blok HEM, Paauw A, Fluit AC, Verhoef J. Evidence of extensive interspecies transfer of integron-mediated antimicrobial resistance genes among multidrug-resistant Enterobacteriaceae in a clinical setting. J Infect Dis. 186:49–56. 2002.12). Leverstein-van Hall MA, Fluit AC, Blok HE, Box AT, Peters ED, Weersink AJ, Verhoef J. Control of nosocomial multiresistant Enterobacteriaceae using a temporary restrictive antibiotic agent policy. Eur J Clin Microbiol Infect Dis. 20:785–791. 2001.13). Leverstein-van Hall MA, Paauw A, Box ATA, Blok HEM, Verhoef J, Fluit AC. Presence of integrons-associated with resistance in the community is widespread and contributes to multidrug resistance in the hospital. J Clin Microbiol. 40:3038–3040. 2002.14). Martinez-Freijo P, Fluit AC, Schmitz FJ, Grek VSC, Verhoef J, Jones ME. Class 1 integrons in Gram-negative isolates from different European hospitals and association with decreased susceptibility to multiple antibiotic compounds. J Antimicrob Chemother. 42:689–696. 1998.15). National Committee for Clinical Laboratory Standards. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically-Sixth Edition. Approved Standard M7-A6. National Committee for Clinical Laboratory Standards. Wayne, PA, USA. 2003.16). Nield BS, Holmes AJ, Gillings MR, Recchia GD, Mabbutt BC, Nevalainen KM, Stokes HW. Recovery of new integron classes from environmental DNA. FEMS Microbiol Lett. 195:59–65. 2001.
Article17). Poly MC, Denis F, Courvalin P, Lambert T. Molecular characterization of integrons in Acinetobacter baumannii: description of a hybrid class 2 integron. Antimicrob Agents Chemother. 44:2684–2688. 2000.18). Recchia GD, Hall RM. Gene cassettes-a new class of mobile element. Microbiol. 141:3015–3027. 1995.19). Rowe-Magnus DA, Guerout AM, Mazel D. Bacterial resistance evolution by recruitment of super-integron gene cassettes. Mol Microbiol. 43:1657–1669. 2002.
Article20). Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: a Laboratory Manual. pp. p. 9.34–9.51. 2nd ed.Cold Spring Harbor Laboratory Press;Cold Spring Harbor, New York: 1989.21). White PA, Mclver CJ, Rawlinson WD. Integrons and gene cassettes in the Enterobacteriaceae. Antimicrob Agents Chemother. 45:2658–2661. 2001.22). Yu HS, Lee JC, Kang HY, Jeong YS, Lee EY, Choi CH, Tae SH, Lee YC, Seol SY, Cho DT. Prevalence of dfr genes associated with integrons and dissemination of dfrA17 among urinary isolates of Escherichia coli in Korea. J Antimicrob Chemother. 53:445–450. 2004.23). Yu HS, Lee JC, Kang HY, Ro DW, Chung JY, Jeong YS, Tae SH, Choi CH, Lee EY, Seol SY, Lee YC, Cho DT. Changes in gene cassettes of class 1 integrons among Escherichia coli isolates from urine specimens collected in Korea during the last two decades. J Clin Microbiol. 41:5429–5433. 2003.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Changes in patterns of antimicrobial susceptibility and class 1 integron carriage among Escherichia coli isolates
- Antimicrobial Resistance in Escherichia coli Isolated from Healthy Volunteers of the Community
- Antimicrobial resistance of Escherichia coli isolated from healthy animals during 2010-2012
- Antimicrobial Resistance Patterns and Integron Carriage of Escherichia coli Isolates Causing Community-Acquired Infections in Turkey
- Comparison of Antimicrobial Resistance in
Escherichia coli Strains Isolated From Healthy Poultry and Swine Farm Workers Using Antibiotics in Korea