Int J Stem Cells.
2014 May;7(1):23-29.
Translational Research: Palatal-derived Ecto-mesenchymal Stem Cells from Human Palate: A New Hope for Alveolar Bone and Cranio-Facial Bone Reconstruction
- Affiliations
-
- 1Periodontology, University of Witten, Germany. prof_wolf.grimm@yahoo.de
- 2Periodontology, Syrian Private University - Damascus, Syrian Arab Republic.
- 3Implantology, Implantogy Center Kassel, Germany.
- 4Implantology, Implantology Center Detmold, Germany.
- 5Cell Biology, SE University Budapest, Hungary.
- 6Implantology, Praxisteam Hasslinghausen, Germany.
- 7Stomatology, Stavropol State Medical University, Stavropol, Russia.
Abstract
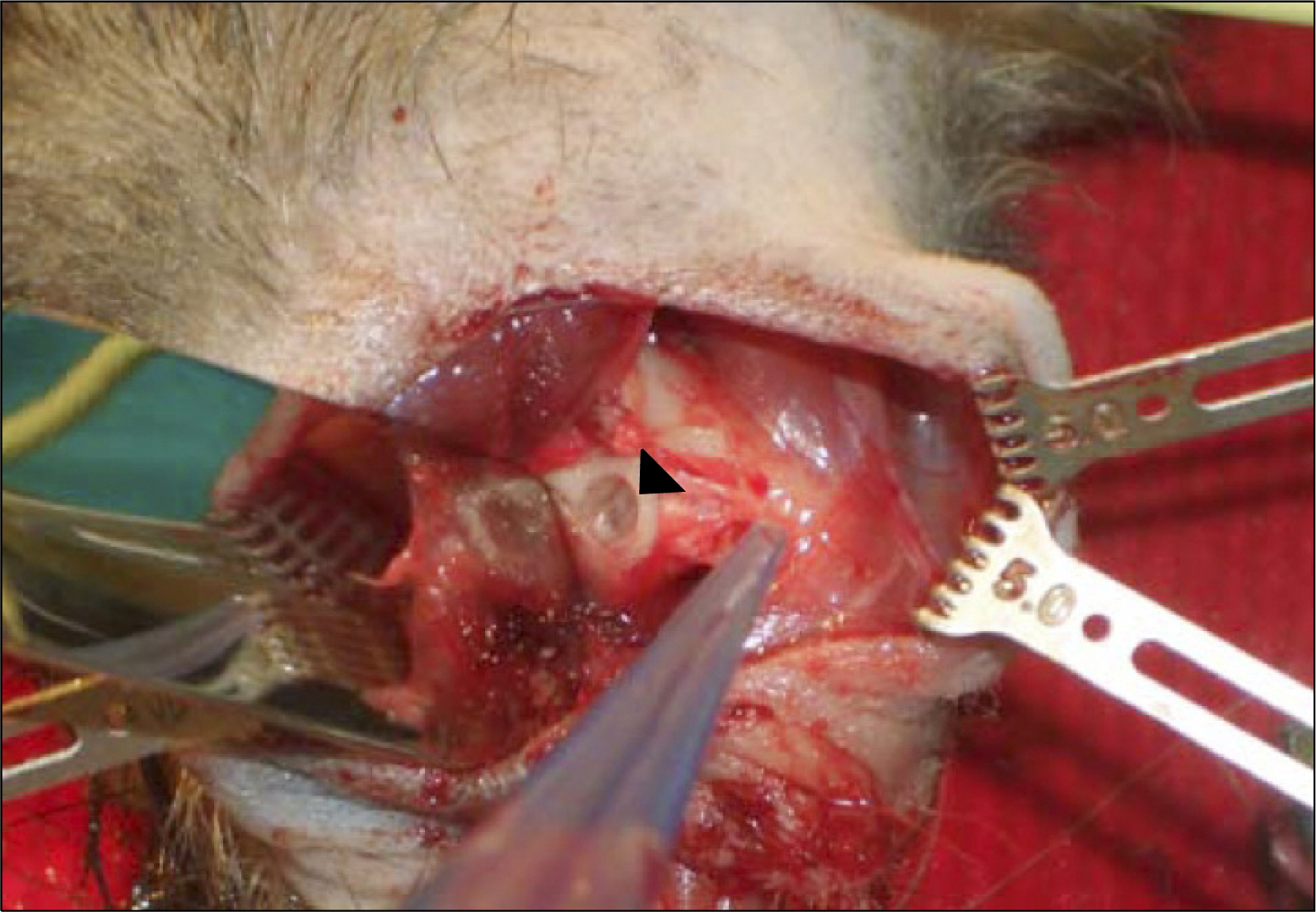
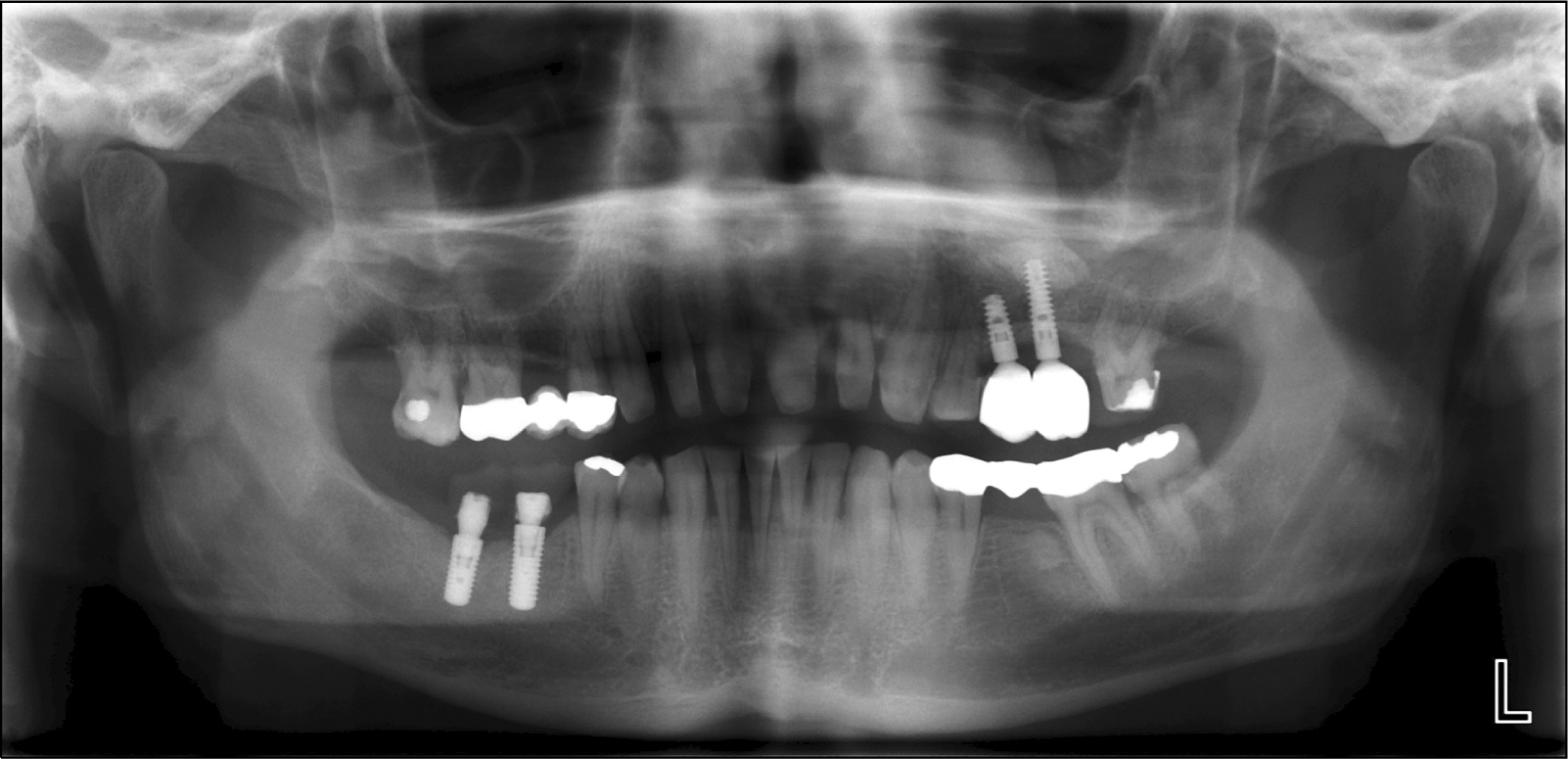
- The management of facial defects has rapidly changed in the last decade. Functional and esthetic requirements have steadily increased along with the refinements of surgery. In the case of advanced atrophy or jaw defects, extensive horizontal and vertical bone augmentation is often unavoidable to enable patients to be fitted with implants. Loss of vertical alveolar bone height is the most common cause for a non primary stability of dental implants in adults. At present, there is no ideal therapeutic approach to cure loss of vertical alveolar bone height and achieve optimal pre-implantological bone regeneration before dental implant placement. Recently, it has been found that specific populations of stem cells and/or progenitor cells could be isolated from different dental resources, namely the dental follicle, the dental pulp and the periodontal ligament. Our research group has cultured palatal-derived stem cells (paldSCs) as dentospheres and further differentiated into various cells of the neuronal and osteogenic lineage, thereby demonstrating their stem cell state. In this publication will be shown whether paldSCs could be differentiated into the osteogenic lineage and, if so, whether these cells are able to regenerate alveolar bone tissue in vivo in an athymic rat model. Furthermore, using these data we have started a proof of principle clinical- and histological controlled study using stem cell-rich palatal tissues for improving the vertical alveolar bone augmentation in critical size defects. The initial results of the study demonstrate the feasibility of using stem cell-mediated tissue engineering to treat alveolar bone defects in humans.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Vinzenz K, Schaudy C. Osteoplastic surgery of the face -state of the art and future aspects. Eur Surg. 2011. 43:1–14.2. Grimm WD, Dittmar T, Varga G, Giesenhagen B. Use of stem-cell induced allogenic bonerings for pre-implanto-logical vertical augmentations of alveolar bone defects in humans- a clinical controlled study. In : Proceedings, BIT’s 5h Annual World Congress of Regenerative Medicine & Stem Cells 2012; December 2–4 2012; Guangzhou, China.3. Grimm WD, Varga G, Dittmar T. Palate-derived ecto-mesenchymal stem cells in Periodontology and Implant Dentistry. Does The Chronically Inflamed Periodontium Harbour Cancer Stem Cells? In : Proceedings, BIT’ 5th Annual World Congress of Regenerative Medicine & Stem Cell 2012; December 2–4 2012; Guangzhou, China.4. Cheng I, Mayle RE, Cox CA, Park DY, Smith RL, Corcoran-Schwartz I, Ponnusamy KE, Oshtory R, Smuck MW, Mitra R, Kharazi AI, Carragee EJ. Functional assessment of the acute local and distal transplantation of human neural stem cells after spinal cord injury. Spine J. 2012. 12:1040–1044.
Article5. Plöger Mathias, Schau I. Kieferkammrekonstruktion mit CAD-CAM-gefertigten allogenen Knochenblöcken. Quintessenz Publishing Company;2010.6. Harris RJ. Successful root coverage: a human histologic evaluation of a case. Int J Periodontics Restorative Dent. 1999. 19:439–447.7. Greiner JF, Hauser S, Widera D, Müller J, Qunneis F, Zander C, Martin I, Mallah J, Schuetzmann D, Prante C, Schwarze H, Prohaska W, Beyer A, Rott K, Hütten A, Gölzhäuser A, Sudhoff H, Kaltschmidt C, Kaltschmidt B. Efficient animal-serum free 3D cultivation method for adult human neural crest-derived stem cell therapeutics. Eur Cell Mater. 2011. 22:403–419.
Article8. Widera D, Zander C, Heidbreder M, Kasperek Y, Noll T, Seitz O, Saldamli B, Sudhoff H, Sader R, Kaltschmidt C, Kaltschmidt B. Adult palatum as a novel source of neural crest-related stem cells. Stem Cells. 2009. 27:1899–1910.
Article9. Heinemann F, Götz W. Oral tissue engineering and implants. Ann Anat. 2012. 194:501.
Article10. Morsczeck C, Petersen J, Völlner F, Driemel O, Reichert T, Beck HC. Proteomic analysis of osteogenic differentiation of dental follicle precursor cells. Electrophoresis. 2009. 30:1175–1184.
Article11. Mao JJ, Giannobile WV, Helms JA, Hollister SJ, Krebsbach PH, Longaker MT, Shi S. Craniofacial tissue engineering by stem cells. J Dent Res. 2006. 85:966–979.
Article12. Schomann T, Qunneis F, Widera D, Kaltschmidt C, Kaltschmidt B. Improved method for ex ovo-cultivation of developing chicken embryos for human stem cell xenografts. Stem Cells In. 2013. 2013:960958.13. Marx RE, Tursun R. A qualitative and quantitative analysis of autologous human multipotent adult stem cells derived from three anatomic areas by marrow aspiration: tibia, anterior ilium, and posterior ilium. Oral Craniofac Tissue Eng. 2011. 1:98–102.
Article14. Arnold WH, Becher S, Dannan A, Widera D, Dittmar T, Jacob M, Mannherz HG, Dittmar T, Kaltschmidt B, Kaltschmidt C, Grimm WD. Morphological characterization of periodontium-derived human stem cells. Ann Anat. 2010. 192:215–219.
Article15. Bartold PM, McCulloch CA, Narayanan AS, Pitaru S. Tissue engineering: a new paradigm for periodontal regeneration based on molecular and cell biology. Periodontol 2000. 2000. 24:253–269.
Article16. Widera D, Grimm WD, Moebius JM, Mikenberg I, Piechaczek C, Gassmann G, Wolff NA, Thévenod F, Kaltschmidt C, Kaltschmidt B. Highly efficient neural differentiation of human somatic stem cells, isolated by minimally invasive periodontal surgery. Stem Cells Dev. 2007. 16:447–460.
Article17. Widera D, Grimm WD, Möbius JM, Dannan A, Becher S, Mikenberg I, Piechaczek C, Gassmann G, Kaltschmidt B. Human periodontium-derived neural stem cells: chance or risk? Regenerative Medicine. 2007. 2:3–61.18. Molnár B, Kádár K, Király M, Porcsalmy B, Somogyi E, Hermann P, Grimm WD, Gera I, Varga G. Isolation, cultivation and characterisation of stem cells in human periodontal ligament. Fogorv Sz. 2008. 101:155–161.19. Techawattanawisal W, Nakahama K, Komaki M, Abe M, Takagi Y, Morita I. Isolation of multipotent stem cells from adult rat periodontal ligament by neurosphere-forming culture system. Biochem Biophys Res Commun. 2007. 357:917–923.
Article20. Király M, Porcsalmy B, Pataki A, Kádár K, Jelitai M, Molnár B, Hermann P, Gera I, Grimm WD, Ganss B, Zsembery A, Varga G. Simultaneous PKC and cAMP activation induces differentiation of human dental pulp stem cells into functionally active neurons. Neurochem Int. 2009. 55:323–332.
Article21. Kharazi A, Levy ML, Visperas MC, Lin CM. Chicken embryonic brain: an in vivo model for verifying neural stem cell potency. J Neurosurg. 2013. 119:512–519.
Article22. Vertelov G, Kharazi L, Muralidhar MG, Sanati G, Tankovich T, Kharazi A. High targeted migration of human mesenchymal stem cells grown in hypoxia is associated with enhanced activation of RhoA. Stem Cell Res Ther. 2013. 4:5.
Article23. Marx RE, Harrell DB. Translational Research: The CD34+ cell is crucial for large-volume bone regeneration from the milieu of bone marrow progenitor cells in craniomandibular reconstruction. Int J Oral Maxillofac Implants. 2014. 29:e201–e209.
Article24. Grimm WD, Dannan A, Becher S, Gassmann G, Arnold W, Varga G, Dittmar T. The ability of human periodontium-derived stem cells to regenerate periodontal tissues: a preliminary in vivo investigation. Int J Periodontics Restorative Dent. 2011. 31:e94–e101.25. Keeve PL, Dittmar T, Gassmann G, Grimm WD, Niggemann B, Friedmann A. Characterization and analysis of migration patterns of dentospheres derived from periodontal tissue and the palate. J Periodontal Res. 2013. 48:276–285.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Bone Marrow-Derived Mesenchymal Stem Cells for Regenerative Medicine
- Management of Alveolar Cleft
- Bone Regeneration from Adipose Stem Cells
- Use of stem cells in bone regeneration in cleft palate patients: review and recommendations
- Concise Review: Differentiation of Human Adult Stem Cells Into Hepatocyte-like Cells In vitro