Lab Anim Res.
2015 Jun;31(2):78-85. 10.5625/lar.2015.31.2.78.
Tumor necrosis factor-alpha deficiency impairs host defense against Streptococcus pneumoniae
- Affiliations
-
- 1Department of Laboratory Animal Medicine, College of Veterinary Medicine, Konkuk University, Seoul, Korea. euisuklove@hanmail.net
- 2Laboratory Animal Facility, Korea Institute of Radiological and Medical Sciences, Seoul, Korea.
- 3Asan Institute for Life Sciences, University of Ulsan College of Medicine, Seoul, Korea.
- 4Laboratory Animal Center, Daegu-Gyeongbuk Medical Innovation Foundation, Daegu, Korea.
- KMID: 2053681
- DOI: http://doi.org/10.5625/lar.2015.31.2.78
Abstract
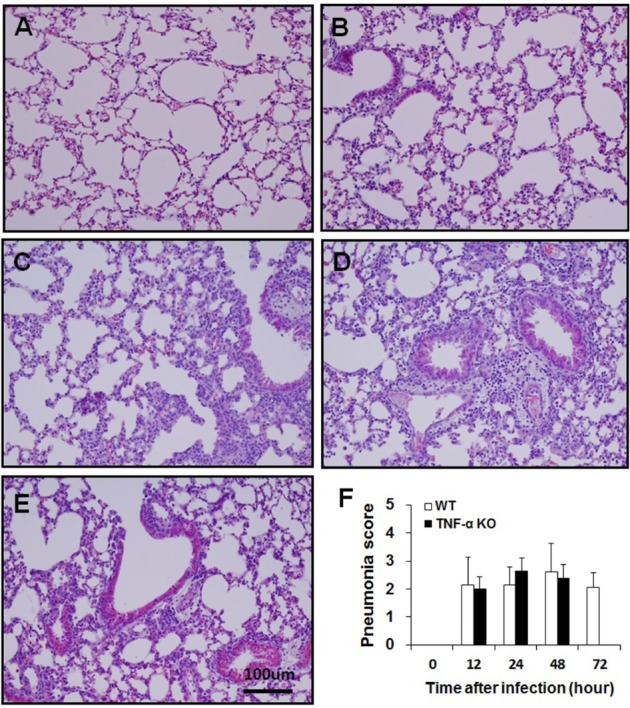
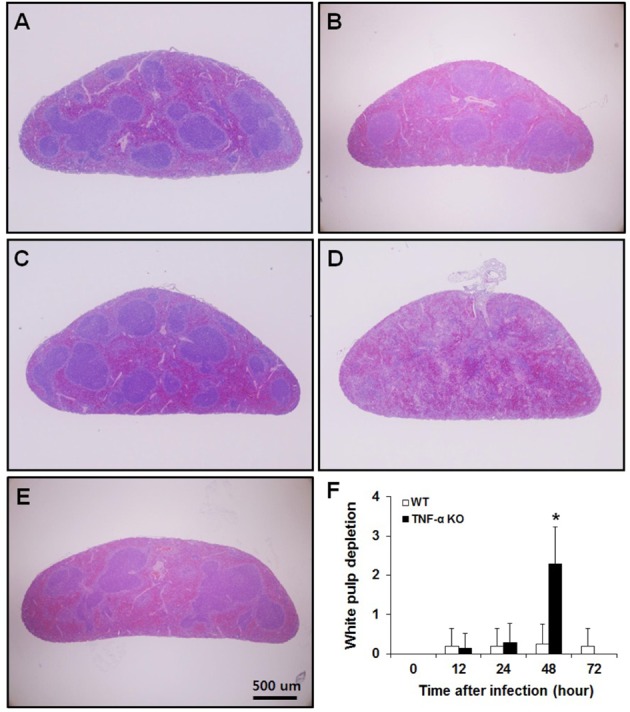
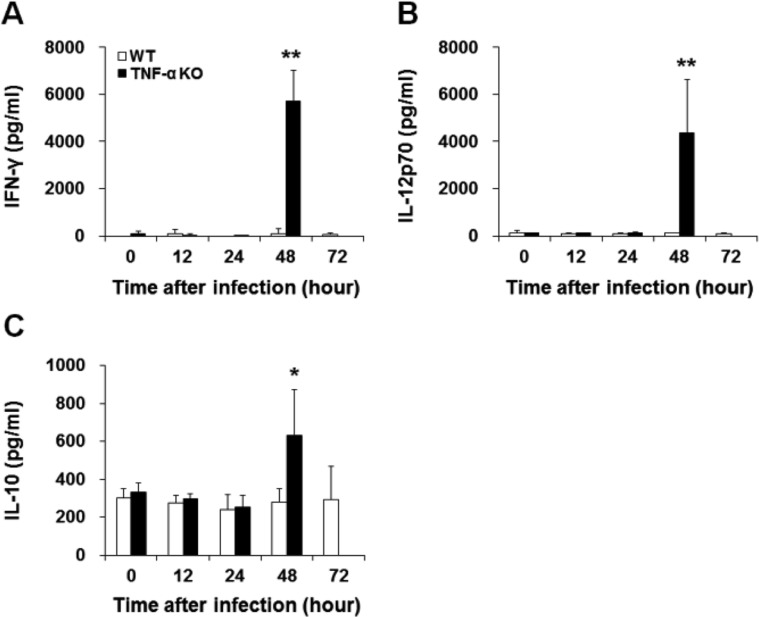
- Streptococcus pneumoniae is a major human pathogen that is involved in community-acquired pneumonia. Tumor necrosis factor-alpha (TNF-alpha) is a pro-inflammatory cytokine that activates immune responses against infection, invasion, injury, or inflammation. To study the role of TNF-alpha during S. pneumoniae infection, a murine pneumococcal pneumonia model was used. We intranasally infected C57BL/6J wild-type (WT) and TNF-alpha knockout (KO) mice with S. pneumoniae D39 serotype 2. In TNF-alpha KO mice, continuous and distinct loss of body weight, and low survival rates were observed. Bacterial counts in the lungs and blood of TNF-alpha KO mice were significantly higher than those in WT mice. Histopathological lesions in the spleen of TNF-alpha KO mice were more severe than those in WT mice. In TNF-alpha KO mice, severe depletion of white pulp was observed and the number of apoptotic cells was significantly increased. Interferon-gamma (IFN-gamma), IL-12p70 and IL-10 levels in serum were significantly increased in TNF-alpha KO mice. TNF-alpha is clearly involved in the regulation of S. pneumoniae infections. Early death and low survival rates of TNF-alpha KO mice were likely caused by a combination of impaired bacterial clearance and damage to the spleen. Our findings suggest that TNF-alpha plays a critical role in protecting the host from systemic S. pneumoniae infection.
MeSH Terms
Figure
Reference
-
1. García-Suárez Mdel M, Cima-Cabal MD, Flórez N, García P, Cernuda-Cernuda R, Astudillo A, Vázquez F, De los Toyos JR, Méndez FJ. Protection against pneumococcal pneumonia in mice by monoclonal antibodies to pneumolysin. Infect Immun. 2004; 72(8):4534–4540. PMID: 15271913.
Article2. O'Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, Lee E, Mulholland K, Levine OS, Cherian T. Hib and Pneumococcal Global Burden of Disease Study Team. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009; 374(9693):893–902. PMID: 19748398.3. Tracey KJ, Cerami A. Tumor necrosis factor, other cytokines and disease. Annu Rev Cell Biol. 1993; 9:317–343. PMID: 8280464.
Article4. Chen G, Goeddel DV. TNF-R1 signaling: a beautiful pathway. Science. 2002; 296(5573):1634–1635. PMID: 12040173.
Article5. Hatta M, Yamamoto N, Miyazato A, Ishii N, Nakamura K, Inden K, Aoyagi T, Kunishima H, Hirakata Y, Suzuki K, Kaku M, Kawakami K. Early production of tumor necrosis factor-alpha by Gr-1 cells and its role in the host defense to pneumococcal infection in lungs. FEMS Immunol Med Microbiol. 2010; 58(2):182–192. PMID: 19909342.6. Takashima K, Tateda K, Matsumoto T, Iizawa Y, Nakao M, Yamaguchi K. Role of tumor necrosis factor alpha in pathogenesis of pneumococcal pneumonia in mice. Infect Immun. 1997; 65(1):257–260. PMID: 8975920.
Article7. Boelen A, Kwakkel J, Wieland CW, St Germain DL, Fliers E, Hernandez A. Impaired bacterial clearance in type 3 deiodinase-deficient mice infected with Streptococcus pneumoniae. Endocrinology. 2009; 150(4):1984–1990. PMID: 19036878.
Article8. Flynn JL, Goldstein MM, Chan J, Triebold KJ, Pfeffer K, Lowenstein CJ, Schreiber R, Mak TW, Bloom BR. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995; 2(6):561–572. PMID: 7540941.9. Kerr AR, Irvine JJ, Search JJ, Gingles NA, Kadioglu A, Andrew PW, McPheat WL, Booth CG, Mitchell TJ. Role of inflammatory mediators in resistance and susceptibility to pneumococcal infection. Infect Immun. 2002; 70(3):1547–1557. PMID: 11854243.
Article10. Baghai M, Osmon DR, Wolk DM, Wold LE, Haidukewych GJ, Matteson EL. Fatal sepsis in a patient with rheumatoid arthritis treated with etanercept. Mayo Clin Proc. 2001; 76(6):653–656. PMID: 11393506.
Article11. Ellerin T, Rubin RH, Weinblatt ME. Infections and anti-tumor necrosis factor alpha therapy. Arthritis Rheum. 2003; 48(11):3013–3022. PMID: 14613261.12. Schneeweiss S, Setoguchi S, Weinblatt ME, Katz JN, Avorn J, Sax PE, Levin R, Solomon DH. Anti-tumor necrosis factor alpha therapy and the risk of serious bacterial infections in elderly patients with rheumatoid arthritis. Arthritis Rheum. 2007; 56(6):1754–1764. PMID: 17530704.13. Houldsworth S, Andrew PW, Mitchell TJ. Pneumolysin stimulates production of tumor necrosis factor alpha and interleukin-1 beta by human mononuclear phagocytes. Infect Immun. 1994; 62(4):1501–1503. PMID: 8132361.
Article14. Rayner CF, Jackson AD, Rutman A, Dewar A, Mitchell TJ, Andrew PW, Cole PJ, Wilson R. Interaction of pneumolysin-sufficient and -deficient isogenic variants of Streptococcus pneumoniae with human respiratory mucosa. Infect Immun. 1995; 63(2):442–447. PMID: 7822008.
Article15. Benton KA, Everson MP, Briles DE. A pneumolysin-negative mutant of Streptococcus pneumoniae causes chronic bacteremia rather than acute sepsis in mice. Infect Immun. 1995; 63(2):448–455. PMID: 7822009.
Article16. Jones MR, Simms BT, Lupa MM, Kogan MS, Mizgerd JP. Lung NF-kappaB activation and neutrophil recruitment require IL-1 and TNF receptor signaling during pneumococcal pneumonia. J Immunol. 2005; 175(11):7530–7535. PMID: 16301661.17. Kirby AC, Raynes JG, Kaye PM. The role played by tumor necrosis factor during localized and systemic infection with Streptococcus pneumoniae. J Infect Dis. 2005; 191(9):1538–1547. PMID: 15809914.18. Kadioglu A, Andrew PW. The innate immune response to pneumococcal lung infection: the untold story. Trends Immunol. 2004; 25(3):143–149. PMID: 15036042.
Article19. Tinsley KW, Grayson MH, Swanson PE, Drewry AM, Chang KC, Karl IE, Hotchkiss RS. Sepsis induces apoptosis and profound depletion of splenic interdigitating and follicular dendritic cells. J Immunol. 2003; 171(2):909–914. PMID: 12847261.
Article20. Schoenborn JR, Wilson CB. Regulation of interferon-gamma during innate and adaptive immune responses. Adv Immunol. 2007; 96:41–101. PMID: 17981204.21. Kaliński P, Hilkens CM, Snijders A, Snijdewint FG, Kapsenberg ML. IL-12-deficient dendritic cells, generated in the presence of prostaglandin E2, promote type 2 cytokine production in maturing human naive T helper cells. J Immunol. 1997; 159(1):28–35. PMID: 9200435.22. Couper KN, Blount DG, Riley EM. IL-10: the master regulator of immunity to infection. J Immunol. 2008; 180(9):5771–5777. PMID: 18424693.
Article23. Rubins JB, Pomeroy C. Role of gamma interferon in the pathogenesis of bacteremic pneumococcal pneumonia. Infect Immun. 1997; 65(7):2975–2977. PMID: 9199475.
Article24. Puddu P, Fantuzzi L, Borghi P, Varano B, Rainaldi G, Guillemard E, Malorni W, Nicaise P, Wolf SF, Belardelli F, Gessani S. IL-12 induces IFN-gamma expression and secretion in mouse peritoneal macrophages. J Immunol. 1997; 159(7):3490–3497. PMID: 9317148.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Difference in Resistance to Streptococcus pneumoniae Infection in Mice
- Serum erythropoietin and tumor necrosis factor ?in neoplasms, chronic inflammatory disorders, and iron deficiency anemias
- Patients treated with a tumor necrosis factor-alpha inhibitor are more likely to develop extrapulmonary tuberculosis
- A Case of Purpura fulminans Caused by Streptococcus pneumoniae
- Antibiotic-Resistant Streptococcus pneumoniae