Lab Anim Res.
2011 Jun;27(2):91-98. 10.5625/lar.2011.27.2.91.
Difference in Resistance to Streptococcus pneumoniae Infection in Mice
- Affiliations
-
- 1Department of Laboratory Animal Medicine, College of Veterinary Medicine, Konkuk University, Seoul, Republic of Korea. yangkyuc@konkuk.ac.kr
- 2Asan Institute for Life Sciences, University of Ulsan College of Medicine, Seoul, Republic of Korea.
- KMID: 2053657
- DOI: http://doi.org/10.5625/lar.2011.27.2.91
Abstract
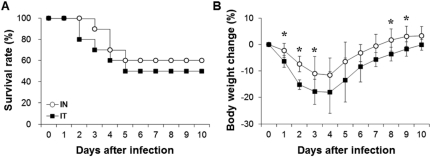
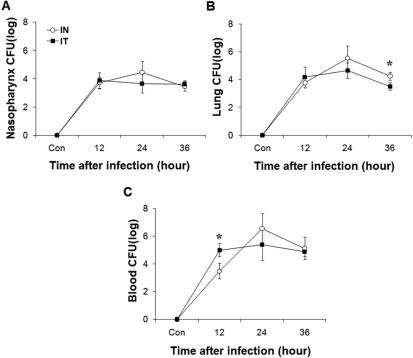
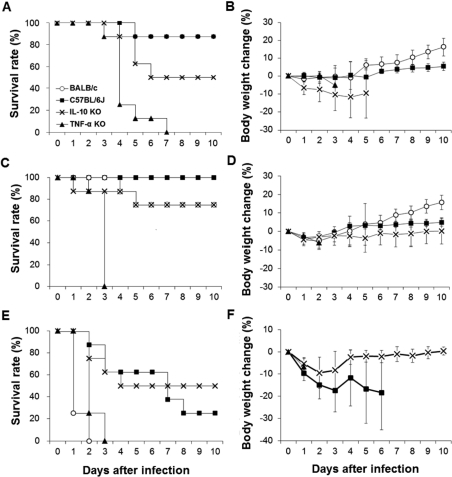
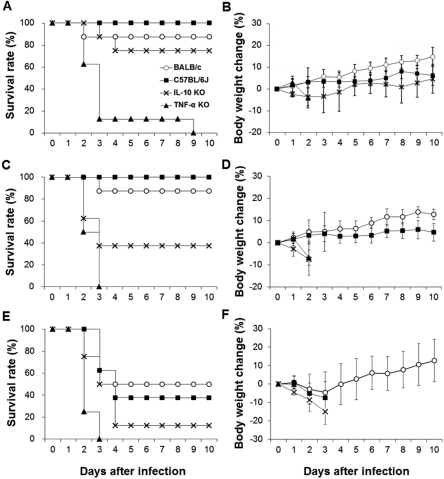
- Streptococcus pneumoniae is a major pathogen that causes various diseases, including pneumonia and sepsis, as millions of people suffer from S. pneumoniae infection worldwide. To better understand the immune and inflammatory responses to S. pneumoniae, we produced murine models. To investigate the differences between intranasal and intratracheal infection, BALB/c mice were infected with S. pneumoniae D39 intranasally or intratracheally. Mice showed no significant differences in survival rates, body weight changes, and bacterial loads. To investigate resistance and susceptibility among mouse strains, BALB/c, C57BL/6J, tumor necrosis factor-alpha (TNF-alpha) knockout, and interleukin-10 (IL-10) knockout mice were infected with S. pneumoniae D39 via intranasal or intravenous routes. In this study, BALB/c and C57BL/6J mice were resistant, IL-10 knockout mice were intermediate, and TNF-alpha knokout mice were susceptible to S. pneumoniae infection. These data show that intranasal and intratracheal infection induced similar results after S. pneumoniae infection, and the genetic background of mice must be considered when studying S. pneumoniae infection in vivo.
MeSH Terms
Figure
Reference
-
1. Kadioglu A, Andrew PW. The innate immune response to pneumococcal lung infection: the untold story. Trends Immunol. 2004; 25(3):143–149. PMID: 15036042.
Article2. O'Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, Lee E, Mulholland K, Levine OS, Cherian T. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009; 374(9693):893–902. PMID: 19748398.3. Finkelstein JA, Huang SS, Daniel J, Rifas-Shiman SL, Kleinman K, Goldmann D, Pelton SI, DeMaria A, Platt R. Antibiotic-resistant Streptococcus pneumoniae in the heptavalent pneumococcal conjugate vaccine era: predictors of carriage in a multicommunity sample. Pediatrics. 2003; 112(4):862–869. PMID: 14523178.4. Huang SS, Hinrichsen VL, Stevenson AE, Rifas-Shiman SL, Kleinman K, Pelton SI, Lipsitch M, Hanage WP, Lee GM, Finkelstein JA. Continued impact of pneumococcal conjugate vaccine on carriage in young children. Pediatrics. 2009; 124(1):e1–e11. PMID: 19564254.
Article5. Liñares J, Ardanuy C, Pallares R, Fenoll A. Changes in antimicrobial resistance, serotypes and genotypes in Streptococcus pneumoniae over a 30-year period. Clin Microbiol Infect. 2010; 16(5):402–410. PMID: 20132251.6. Chiavolini D, Pozzi G, Ricci S. Animal models of Streptococcus pneumoniae disease. Clin Microbiol Rev. 2008; 21(4):666–685. PMID: 18854486.7. Gingles NA, Alexander JE, Kadioglu A, Andrew PW, Kerr A, Mitchell TJ, Hopes E, Denny P, Brown S, Jones HB, Little S, Booth GC, McPheat WL. Role of genetic resistance in invasive pneumococcal infection: identification and study of susceptibility and resistance in inbred mouse strains. Infect Immun. 2001; 69(1):426–434. PMID: 11119534.
Article8. Mizrachi-Nebenzahl Y, Lifshitz S, Teitelbaum R, Novick S, Levi A, Benharroch D, Ling E, Dagan R. Differential activation of the immune system by virulent Streptococcus pneumoniae strains determines recovery or death of the host. Clin Exp Immunol. 2003; 134(1):23–31. PMID: 12974750.9. Benton KA, Paton JC, Briles DE. The hemolytic and complement-activating properties of pneumolysin do not contribute individually to virulence in a pneumococcal bacteremia model. Microb Pathog. 1997; 23(4):201–209. PMID: 9344781.
Article10. Standiford TJ, Kunkel SL, Greenberger MJ, Laichalk LL, Strieter RM. Expression and regulation of chemokines in bacterial pneumonia. J Leukoc Biol. 1996; 59(1):24–28. PMID: 8558063.
Article11. Klebanoff SJ, Vadas MA, Harlan JM, Sparks LH, Gamble JR, Agosti JM, Waltersdorph AM. Stimulation of neutrophils by tumor necrosis factor. J Immunol. 1986; 136(11):4220–4225. PMID: 3009619.12. Sriskandan S, Cohen J. Gram-positive sepsis. Mechanisms and differences from gram-negative sepsis. Infect Dis Clin North Am. 1999; 13(2):397–412. PMID: 10340174.13. van der Poll T, Marchant A, Keogh CV, Goldman M, Lowry SF. Interleukin-10 impairs host defense in murine pneumococcal pneumonia. J Infect Dis. 1996; 174(5):994–1000. PMID: 8896500.14. Rubins JB, Charboneau D, Fasching C, Berry AM, Paton JC, Alexander JE, Andrew PW, Mitchell TJ, Janoff EN. Distinct roles for pneumolysin's cytotoxic and complement activities in the pathogenesis of pneumococcal pneumonia. Am J Respir Crit Care Med. 1996; 153(4 Pt 1):1339–1346. PMID: 8616564.
Article15. Takashima K, Tateda K, Matsumoto T, Iizawa Y, Nakao M, Yamaguchi K. Role of tumor necrosis factor alpha in pathogenesis of pneumococcal pneumonia in mice. Infect Immun. 1997; 65(1):257–260. PMID: 8975920.
Article16. O'Brien DP, Briles DE, Szalai AJ, Tu AH, Sanz I, Nahm MH. Tumor necrosis factor alpha receptor I is important for survival from Streptococcus pneumoniae infections. Infect Immun. 1999; 67(2):595–601. PMID: 9916064.17. Rijneveld AW, Florquin S, Branger J, Speelman P, Van Deventer SJ, van der Poll T. TNF-alpha compensates for the impaired host defense of IL-1 type I receptor-deficient mice during pneumococcal pneumonia. J Immunol. 2001; 167(9):5240–5246. PMID: 11673538.18. Hatta M, Yamamoto N, Miyazato A, Ishii N, Nakamura K, Inden K, Aoyagi T, Kunishima H, Hirakata Y, Suzuki K, Kaku M, Kawakami K. Early production of tumor necrosis factor-alpha by Gr-1 cells and its role in the host defense to pneumococcal infection in lungs. FEMS Immunol Med Microbiol. 2010; 58(2):182–192. PMID: 19909342.19. van der Poll T, Keogh CV, Buurman WA, Lowry SF. Passive immunization against tumor necrosis factor-alpha impairs host defense during pneumococcal pneumonia in mice. Am J Respir Crit Care Med. 1997; 155(2):603–608. PMID: 9032201.
Article20. Sanjabi S, Zenewicz LA, Kamanaka M, Flavell RA. Anti-inflammatory and pro-inflammatory roles of TGF-beta, IL-10, and IL-22 in immunity and autoimmunity. Curr Opin Pharmacol. 2009; 9(4):447–453. PMID: 19481975.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antibiotic-Resistant Streptococcus pneumoniae
- Penicillin resistance in streptococcus pneumoniae
- Clinical Implications of Drug-Resistant Streptococcus pneumoniae as a Cause of Community Acquired Pneumonia
- Comparison of the virulence of Streptococcus pneumoniae in ICR mouse stocks of three different origins
- Two Cases of Meningitis Caused by Penicillin-and Cephalosporin-resistant Streptococcus pneumoniae