Tuberc Respir Dis.
2012 Nov;73(5):258-265. 10.4046/trd.2012.73.5.258.
Inhibition of Vitamin D Receptor Translocation by Cigarette Smoking Extracts
- Affiliations
-
- 1Department of Internal Medicine, Soonchunhyang University Hospital, Soonchunhyang University College of Medicine, Seoul, Korea.
- 2Department of Internal Medicine, Soonchunhyang University Bucheon Hospital, Soonchunhyang University College of Medicine, Bucheon, Korea. mdcspark@unitel.co.kr
- 3Genome Research Center for Allergy and Respiratory Diseases, Soonchunhyang University Bucheon Hospital, Soonchunhyang University College of Medicine, Bucheon, Korea.
- 4Department of Internal Medicine, Soonchunhyang University Cheoan Hospital, Soonchunhyang University College of Medicine, Cheonan, Korea.
- KMID: 2050678
- DOI: http://doi.org/10.4046/trd.2012.73.5.258
Abstract
- BACKGROUND
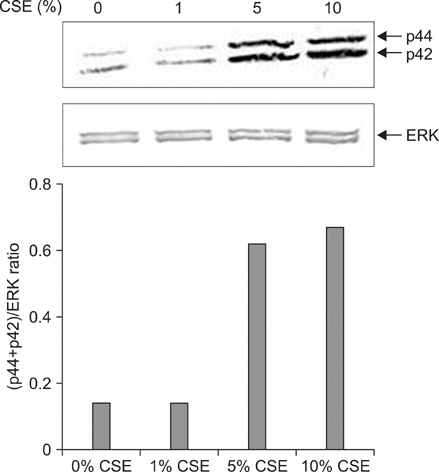
Vitamin D can translocate a vitamin D receptor (VDR) from the nucleus to the cell membranes. The meaning of this translocation is not elucidated in terms of a role in pathogenesis of chronic obstructive pulmonary disease (COPD) till now. VDR deficient mice are prone to develop emphysema, suggesting that abnormal function of VDR might influence a generation of COPD. The blood levels of vitamin D have known to be well correlated with that of lung function in patients with COPD, and smoking is the most important risk factor in development of COPD. This study was performed to investigate whether cigarette smoke extracts (CSE) can inhibit the translocation of VDR and whether mitogen activated protein kinases (MAPKs) are involved in this inhibition.
METHODS
Human alveolar basal epithelial cell line (A549) was used in this study. 1,25-(OH2)D3 and/or MAPKs inhibitors and antioxidants were pre-incubated before stimulation with 10% CSE, and then nucleus and microsomal proteins were extracted for a Western blot of VDR.
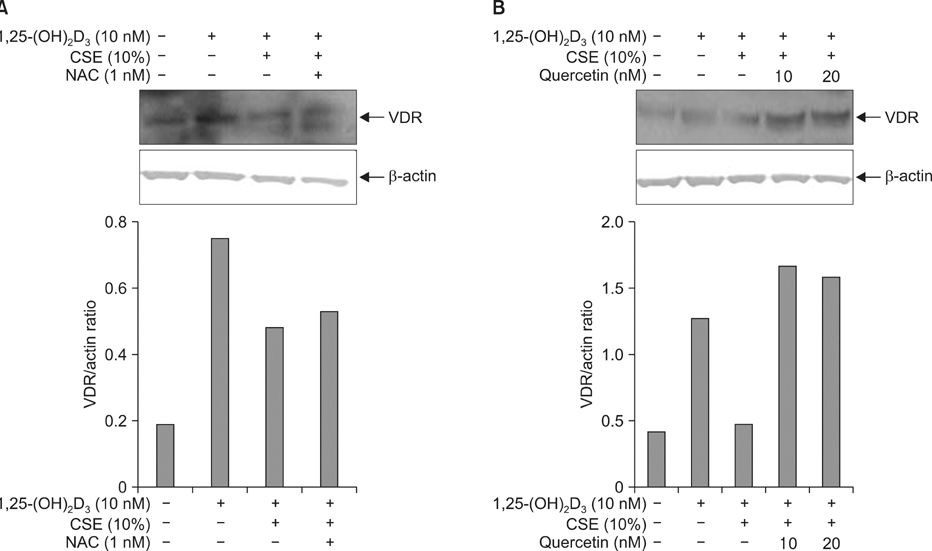
RESULTS
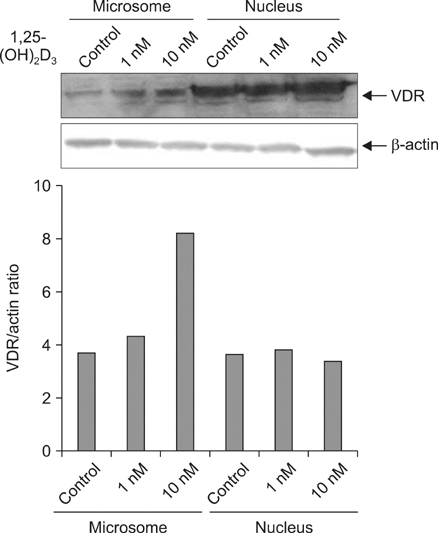
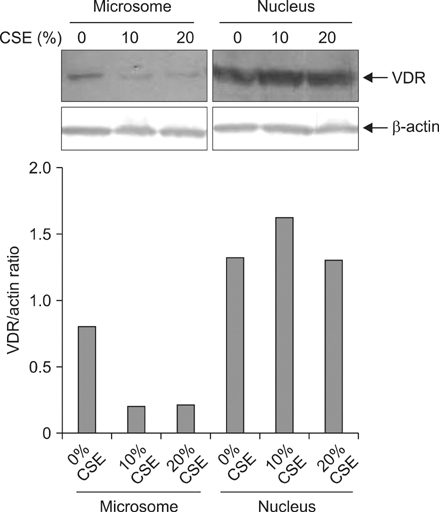
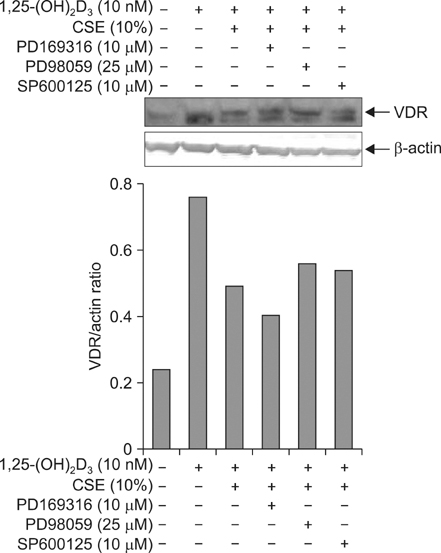
Five minutes treatment of 1,25-(OH2)D3 induced translocation of VDR from nucleus to microsomes by a dose-dependent manner. CSE inhibited 1,25-(OH2)D3-induced translocation of VDR in both concentrations of 10% and 20%. All MAPKs inhibitors did not suppress the inhibitory effects of CSE on the 1,25-(OH2)D3-induced translocation of VDR. Quercetin suppressed the inhibitory effects of CSE on the 1,25-(OH2)D3-induced translocation of VDR, but not in n-acetylcysteine.
CONCLUSION
CSE has an ability to inhibit vitamin D-induced VDR translocation, but MAPKs are not involved in this inhibition.
Keyword
MeSH Terms
-
Animals
Antioxidants
Blotting, Western
Cell Membrane
Emphysema
Epithelial Cells
Humans
Lung
Mice
Microsomes
Mitogen-Activated Protein Kinases
Proteins
Pulmonary Disease, Chronic Obstructive
Quercetin
Receptors, Calcitriol
Risk Factors
Smoke
Smoking
Tobacco Products
Vitamin D
Vitamins
Antioxidants
Mitogen-Activated Protein Kinases
Proteins
Quercetin
Receptors, Calcitriol
Smoke
Vitamin D
Vitamins
Figure
Cited by 1 articles
-
The Phosphodiesterase 4 Inhibitor Roflumilast Protects against Cigarette Smoke Extract-Induced Mitophagy-Dependent Cell Death in Epithelial Cells
Sun Young Kyung, Yu Jin Kim, Eun Suk Son, Sung Hwan Jeong, Jeong-Woong Park
Tuberc Respir Dis. 2018;81(2):138-147. doi: 10.4046/trd.2017.0115.
Reference
-
1. Hughes DA, Norton R. Vitamin D and respiratory health. Clin Exp Immunol. 2009. 158:20–25.2. Sutherland ER, Goleva E, Jackson LP, Stevens AD, Leung DY. Vitamin D levels, lung function, and steroid response in adult asthma. Am J Respir Crit Care Med. 2010. 181:699–704.3. Black PN, Scragg R. Relationship between serum 25-hydroxyvitamin d and pulmonary function in the third national health and nutrition examination survey. Chest. 2005. 128:3792–3798.4. Janssens W, Bouillon R, Claes B, Carremans C, Lehouck A, Buysschaert I, et al. Vitamin D deficiency is highly prevalent in COPD and correlates with variants in the vitamin D-binding gene. Thorax. 2010. 65:215–220.5. Shaheen SO, Jameson KA, Robinson SM, Boucher BJ, Syddall HE, Sayer AA, et al. Relationship of vitamin D status to adult lung function and COPD. Thorax. 2011. 66:692–698.6. Ting HJ, Bao BY, Reeder JE, Messing EM, Lee YF. Increased expression of corepressors in aggressive androgen-independent prostate cancer cells results in loss of 1alpha,25-dihydroxyvitamin D3 responsiveness. Mol Cancer Res. 2007. 5:967–980.7. Buitrago C, Boland R. Caveolae and caveolin-1 are implicated in 1alpha,25(OH)2-vitamin D3-dependent modulation of Src, MAPK cascades and VDR localization in skeletal muscle cells. J Steroid Biochem Mol Biol. 2010. 121:169–175.8. Capiati D, Benassati S, Boland RL. 1,25(OH)2-vitamin D3 induces translocation of the vitamin D receptor (VDR) to the plasma membrane in skeletal muscle cells. J Cell Biochem. 2002. 86:128–135.9. Brot C, Jorgensen NR, Sorensen OH. The influence of smoking on vitamin D status and calcium metabolism. Eur J Clin Nutr. 1999. 53:920–926.10. Smelter DF, Sathish V, Thompson MA, Pabelick CM, Vassallo R, Prakash YS. Thymic stromal lymphopoietin in cigarette smoke-exposed human airway smooth muscle. J Immunol. 2010. 185:3035–3040.11. Sundar IK, Hwang JW, Wu S, Sun J, Rahman I. Deletion of vitamin D receptor leads to premature emphysema/COPD by increased matrix metalloproteinases and lymphoid aggregates formation. Biochem Biophys Res Commun. 2011. 406:127–133.12. Gallieni M, Cozzolino M, Fallabrino G, Pasho S, Olivi L, Brancaccio D. Vitamin D: physiology and pathophysiology. Int J Artif Organs. 2009. 32:87–94.13. Bernert JT, Jacob P 3rd, Holiday DB, Benowitz NL, Sosnoff CS, Doig MV, et al. Interlaboratory comparability of serum cotinine measurements at smoker and nonsmoker concentration levels: a round-robin study. Nicotine Tob Res. 2009. 11:1458–1466.14. Clunes LA, Bridges A, Alexis N, Tarran R. In vivo versus in vitro airway surface liquid nicotine levels following cigarette smoke exposure. J Anal Toxicol. 2008. 32:201–207.15. Shen N, Gong T, Wang JD, Meng FL, Qiao L, Yang RL, et al. Cigarette smoke-induced pulmonary inflammatory responses are mediated by EGR-1/GGPPS/MAPK signaling. Am J Pathol. 2011. 178:110–118.16. Springer J, Scholz FR, Peiser C, Groneberg DA, Fischer A. SMAD-signaling in chronic obstructive pulmonary disease: transcriptional down-regulation of inhibitory SMAD 6 and 7 by cigarette smoke. Biol Chem. 2004. 385:649–653.17. Shih RH, Lee IT, Hsieh HL, Kou YR, Yang CM. Cigarette smoke extract induces HO-1 expression in mouse cerebral vascular endothelial cells: involvement of c-Src/NADPH oxidase/PDGFR/JAK2/STAT3 pathway. J Cell Physiol. 2010. 225:741–750.18. Bischoff SC. Quercetin: potentials in the prevention and therapy of disease. Curr Opin Clin Nutr Metab Care. 2008. 11:733–740.19. Perez-Vizcaino F, Bishop-Bailley D, Lodi F, Duarte J, Cogolludo A, Moreno L, et al. The flavonoid quercetin induces apoptosis and inhibits JNK activation in intimal vascular smooth muscle cells. Biochem Biophys Res Commun. 2006. 346:919–925.20. Edwards RL, Lyon T, Litwin SE, Rabovsky A, Symons JD, Jalili T. Quercetin reduces blood pressure in hypertensive subjects. J Nutr. 2007. 137:2405–2411.21. Rivera L, Morón R, Sánchez M, Zarzuelo A, Galisteo M. Quercetin ameliorates metabolic syndrome and improves the inflammatory status in obese Zucker rats. Obesity (Silver Spring). 2008. 16:2081–2087.22. Inoue J, Choi JM, Yoshidomi T, Yashiro T, Sato R. Quercetin enhances VDR activity, leading to stimulation of its target gene expression in Caco-2 cells. J Nutr Sci Vitaminol (Tokyo). 2010. 56:326–330.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Associations between the Frequency of Electronic Cigarette Use and Smoking-related Characteristics among Adolescent Smokers
- Cigarette Smoking in South Korea: A Narrative Review
- Effects of Cigarette Smoking on Blood Flow and Structural Change in Rat Vagina
- Habits of smoking and pulmonary function in current smokers
- Effect of Smoking on the Levels of Antioxidant Vitamins and Enzymes in Healthy and Young Men