Ann Pediatr Endocrinol Metab.
2013 Jun;18(2):45-54. 10.6065/apem.2013.18.2.45.
The pleiomorphic actions of vitamin D and its importance for children
- Affiliations
-
- 1Department of Pediatrics, Bundang Jesaeng General Hospital, Daejin Medical Center, Seongnam, Korea. odajulia@dmc.or.kr
- KMID: 2047460
- DOI: http://doi.org/10.6065/apem.2013.18.2.45
Abstract
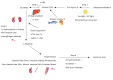
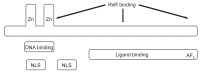
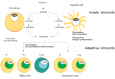
- Knowledge regarding the physiological role and dietary requirements of vitamin D has dramatically expanded over the past several decades. The "new" vitamin D is not only a mediator of calcium homeostasis, it also has important immunomodulatory, antimicrobial, and antiproliferative actions. In spite of the interest in vitamin D as a mediator in many chronic diseases of adulthood such as cancer and type II diabetes, less attention has been given to the implications of the new understanding of vitamin D for child and adolescent health. Recently, rickets caused by vitamin D deficiency is resurging in developed countries. Therefore, pharmacokinetic studies and epidemiologic research that incorporates clinical and functional outcomes are needed to clarify the role of vitamin D in growth and development in Korean children and adolescents and to determine vitamin D dietary requirements.
Keyword
MeSH Terms
Figure
Cited by 3 articles
-
Prevalence of Vitamin D Deficiency and Insufficiency in Korean Children and Adolescents and Associated Factors
Anna Lee, Se Hwi Kim, Chung Mo Nam, Young-Jin Kim, Soo-Ho Joo, Kyoung-Ryul Lee
Lab Med Online. 2016;6(2):70-78. doi: 10.3343/lmo.2016.6.2.70.Association of vitamin D deficiency with clinical outcomes in critically ill Korean children
Won Kyoung Jhang, Da Hyun Kim, Seong Jong Park
Nutr Res Pract. 2020;14(1):12-19. doi: 10.4162/nrp.2020.14.1.12.Serum vitamin D status in children and adolescence with diabetes according to season and age
Sung Su Jung, Min Sun Kim, Dae Yeol Lee
Ann Pediatr Endocrinol Metab. 2014;19(1):13-19. doi: 10.6065/apem.2014.19.1.13.
Reference
-
1. Girgis CM, Clifton-Bligh RJ, Hamrick MW, Holick MF, Gunton JE. The roles of vitamin D in skeletal muscle: form, function, and metabolism. Endocr Rev. 2013; 34:33–83. PMID: 23169676.
Article2. Rosen CJ, Adams JS, Bikle DD, Black DM, Demay MB, Manson JE, et al. The nonskeletal effects of vitamin D: an Endocrine Society scientific statement. Endocr Rev. 2012; 33:456–492. PMID: 22596255.
Article3. Matsuoka LY, Wortsman J, Dannenberg MJ, Hollis BW, Lu Z, Holick MF. Clothing prevents ultraviolet-B radiation-dependent photosynthesis of vitamin D3. J Clin Endocrinol Metab. 1992; 75:1099–1103. PMID: 1328275.
Article4. Andersson S, Jornvall H. Sex differences in cytochrome P-450-dependent 25-hydroxylation of C27-steroids and vitamin D3 in rat liver microsomes. J Biol Chem. 1986; 261:16932–16936. PMID: 3023373.
Article5. Wikvall K. Cytochrome P450 enzymes in the bioactivation of vitamin D to its hormonal form (review). Int J Mol Med. 2001; 7:201–209. PMID: 11172626.
Article6. Shinkyo R, Sakaki T, Kamakura M, Ohta M, Inouye K. Metabolism of vitamin D by human microsomal CYP2R1. Biochem Biophys Res Commun. 2004; 324:451–457. PMID: 15465040.
Article7. Cheng JB, Levine MA, Bell NH, Mangelsdorf DJ, Russell DW. Genetic evidence that the human CYP2R1 enzyme is a key vitamin D 25-hydroxylase. Proc Natl Acad Sci U S A. 2004; 101:7711–7715. PMID: 15128933.
Article8. Omdahl JL, Morris HA, May BK. Hydroxylase enzymes of the vitamin D pathway: expression, function, and regulation. Annu Rev Nutr. 2002; 22:139–166. PMID: 12055341.
Article9. Anderson PH, May BK, Morris HA. Vitamin D metabolism: new concepts and clinical implications. Clin Biochem Rev. 2003; 24:13–26. PMID: 18650961.10. Shinki T, Shimada H, Wakino S, Anazawa H, Hayashi M, Saruta T, et al. Cloning and expression of rat 25-hydroxyvitamin D3-1alpha-hydroxylase cDNA. Proc Natl Acad Sci U S A. 1997; 94:12920–12925. PMID: 9371776.11. Kitanaka S, Takeyama K, Murayama A, Sato T, Okumura K, Nogami M, et al. Inactivating mutations in the 25-hydroxyvitamin D3 1alpha-hydroxylase gene in patients with pseudovitamin D-deficiency rickets. N Engl J Med. 1998; 338:653–661. PMID: 9486994.
Article12. Dardenne O, Prud'homme J, Arabian A, Glorieux FH, St-Arnaud R. Targeted inactivation of the 25-hydroxyvitamin D(3)-1(alpha)-hydroxylase gene (CYP27B1) creates an animal model of pseudovitamin D-deficiency rickets. Endocrinology. 2001; 142:3135–3141. PMID: 11416036.
Article13. Lucas PA, Brown RC, Woodhead JS, Coles GA. 1,25-dihydroxycholecalciferol and parathyroid hormone in advanced chronic renal failure: effects of simultaneous protein and phosphorus restriction. Clin Nephrol. 1986; 25:7–10. PMID: 3754187.14. Demetriou ET, Pietras SM, Holick MF. Hypercalcemia and soft tissue calcification owing to sarcoidosis: the sunlight-cola connection. J Bone Miner Res. 2010; 25:1695–1699. PMID: 20200968.
Article15. Nykjaer A, Dragun D, Walther D, Vorum H, Jacobsen C, Herz J, et al. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell. 1999; 96:507–515. PMID: 10052453.
Article16. Kaseda R, Hosojima M, Sato H, Saito A. Role of megalin and cubilin in the metabolism of vitamin D(3). Ther Apher Dial. 2011; 15(Suppl 1):14–17. PMID: 21595846.
Article17. Brenza HL, DeLuca HF. Regulation of 25-hydroxyvitamin D3 1alpha-hydroxylase gene expression by parathyroid hormone and 1,25-dihydroxyvitamin D3. Arch Biochem Biophys. 2000; 381:143–152. PMID: 11019830.
Article18. Robinson CJ, Spanos E, James MF, Pike JW, Haussler MR, Makeen AM, et al. Role of prolactin in vitamin D metabolism and calcium absorption during lactation in the rat. J Endocrinol. 1982; 94:443–453. PMID: 6896886.
Article19. Pike JW, Spanos E, Colston KW, MacIntyre I, Haussler MR. Influence of estrogen on renal vitamin D hydroxylases and serum 1alpha,25-(OH)2D3 in chicks. Am J Physiol. 1978; 235:E338–E343. PMID: 211856.
Article20. White JH. Regulation of intracrine production of 1,25-dihydroxyvitamin D and its role in innate immune defense against infection. Arch Biochem Biophys. 2012; 523:58–63. PMID: 22107948.
Article21. Anderson PH, O'Loughlin PD, May BK, Morris HA. Quantification of mRNA for the vitamin D metabolizing enzymes CYP27B1 and CYP24 and vitamin D receptor in kidney using real-time reverse transcriptase- polymerase chain reaction. J Mol Endocrinol. 2003; 31:123–132. PMID: 12914530.
Article22. Schlingmann KP, Kaufmann M, Weber S, Irwin A, Goos C, John U, et al. Mutations in CYP24A1 and idiopathic infantile hypercalcemia. N Engl J Med. 2011; 365:410–421. PMID: 21675912.
Article23. Haussler MR, Norman AW. Chromosomal receptor for a vitamin D metabolite. Proc Natl Acad Sci U S A. 1969; 62:155–162. PMID: 5253652.
Article24. McDonnell DP, Mangelsdorf DJ, Pike JW, Haussler MR, O'Malley BW. Molecular cloning of complementary DNA encoding the avian receptor for vitamin D. Science. 1987; 235:1214–1217. PMID: 3029866.
Article25. Baker AR, McDonnell DP, Hughes M, Crisp TM, Mangelsdorf DJ, Haussler MR, et al. Cloning and expression of full-length cDNA encoding human vitamin D receptor. Proc Natl Acad Sci U S A. 1988; 85:3294–3298. PMID: 2835767.
Article26. Walters MR. Newly identified actions of the vitamin D endocrine system. Endocr Rev. 1992; 13:719–764. PMID: 1333949.
Article27. Harris SS, Eccleshall TR, Gross C, Dawson-Hughes B, Feldman D. The vitamin D receptor start codon polymorphism (FokI) and bone mineral density in premenopausal American black and white women. J Bone Miner Res. 1997; 12:1043–1048. PMID: 9200003.
Article28. Rachez C, Gamble M, Chang CP, Atkins GB, Lazar MA, Freedman LP. The DRIP complex and SRC-1/p160 coactivators share similar nuclear receptor binding determinants but constitute functionally distinct complexes. Mol Cell Biol. 2000; 20:2718–2726. PMID: 10733574.
Article29. Makowski A, Brzostek S, Cohen RN, Hollenberg AN. Determination of nuclear receptor corepressor interactions with the thyroid hormone receptor. Mol Endocrinol. 2003; 17:273–286. PMID: 12554754.
Article30. Rochel N, Wurtz JM, Mitschler A, Klaholz B, Moras D. The crystal structure of the nuclear receptor for vitamin D bound to its natural ligand. Mol Cell. 2000; 5:173–179. PMID: 10678179.
Article31. Wurtz JM, Bourguet W, Renaud JP, Vivat V, Chambon P, Moras D, et al. A canonical structure for the ligand-binding domain of nuclear receptors. Nat Struct Biol. 1996; 3:87–94. PMID: 8548460.
Article32. Morley P, Whitfield JF, Vanderhyden BC, Tsang BK, Schwartz JL. A new, nongenomic estrogen action: the rapid release of intracellular calcium. Endocrinology. 1992; 131:1305–1312. PMID: 1505465.
Article33. Caffrey JM, Farach-Carson MC. Vitamin D3 metabolites modulate dihydropyridine-sensitive calcium currents in clonal rat osteosarcoma cells. J Biol Chem. 1989; 264:20265–20274. PMID: 2479647.
Article34. Nemere I, Dormanen MC, Hammond MW, Okamura WH, Norman AW. Identification of a specific binding protein for 1 alpha,25-dihydroxyvitamin D3 in basal-lateral membranes of chick intestinal epithelium and relationship to transcaltachia. J Biol Chem. 1994; 269:23750–23756. PMID: 8089147.
Article35. Viljakainen HT, Saarnio E, Hytinantti T, Miettinen M, Surcel H, Makitie O, et al. Maternal vitamin D status determines bone variables in the newborn. J Clin Endocrinol Metab. 2010; 95:1749–1757. PMID: 20139235.
Article36. Viljakainen HT, Korhonen T, Hytinantti T, Laitinen EK, Andersson S, Makitie O, et al. Maternal vitamin D status affects bone growth in early childhood: a prospective cohort study. Osteoporos Int. 2011; 22:883–891. PMID: 21153404.
Article37. Javaid MK, Crozier SR, Harvey NC, Gale CR, Dennison EM, Boucher BJ, et al. Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: a longitudinal study. Lancet. 2006; 367:36–43. PMID: 16399151.
Article38. Javaid MK, Godfrey KM, Taylor P, Shore SR, Breier B, Arden NK, et al. Umbilical venous IGF-1 concentration, neonatal bone mass, and body composition. J Bone Miner Res. 2004; 19:56–63. PMID: 14753737.
Article39. Ammann P, Shen V, Robin B, Mauras Y, Bonjour JP, Rizzoli R. Strontium ranelate improves bone resistance by increasing bone mass and improving architecture in intact female rats. J Bone Miner Res. 2004; 19:2012–2020. PMID: 15537445.
Article40. Wells JC, Chomtho S, Fewtrell MS. Programming of body composition by early growth and nutrition. Proc Nutr Soc. 2007; 66:423–434. PMID: 17637095.
Article41. Zhou S, LeBoff MS, Glowacki J. Vitamin D metabolism and action in human bone marrow stromal cells. Endocrinology. 2010; 151:14–22. PMID: 19966181.
Article42. Soliman AT, Al Khalaf F, Alhemaidi N, Al Ali M, Al Zyoud M, Yakoot K. Linear growth in relation to the circulating concentrations of insulin-like growth factor I, parathyroid hormone, and 25-hydroxy vitamin D in children with nutritional rickets before and after treatment: endocrine adaptation to vitamin D deficiency. Metabolism. 2008; 57:95–102. PMID: 18078865.
Article43. Earthman CP, Beckman LM, Masodkar K, Sibley SD. The link between obesity and low circulating 25-hydroxyvitamin D concentrations: considerations and implications. Int J Obes (Lond). 2012; 36:387–396. PMID: 21694701.
Article44. Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000; 72:690–693. PMID: 10966885.
Article45. Villamor E, Marin C, Mora-Plazas M, Baylin A. Vitamin D deficiency and age at menarche: a prospective study. Am J Clin Nutr. 2011; 94:1020–1025. PMID: 21831989.
Article46. Dossus L, Allen N, Kaaks R, Bakken K, Lund E, Tjonneland A, et al. Reproductive risk factors and endometrial cancer: the European Prospective Investigation into Cancer and Nutrition. Int J Cancer. 2010; 127:442–451. PMID: 19924816.
Article47. van Lenthe FJ, Kemper CG, van Mechelen W. Rapid maturation in adolescence results in greater obesity in adulthood: the Amsterdam Growth and Health Study. Am J Clin Nutr. 1996; 64:18–24. PMID: 8669409.
Article48. He C, Zhang C, Hunter DJ, Hankinson SE, Buck Louis GM, Hediger ML, et al. Age at menarche and risk of type 2 diabetes: results from 2 large prospective cohort studies. Am J Epidemiol. 2010; 171:334–344. PMID: 20026580.
Article49. Committee to Review Dietary Reference Intakes for Vitamin D and Calcium, Institute of Medicine. Dietary reference intakes for calcium and vitamin D. In : Ross AC, Taylor CL, Yaktine AL, Del Valle HB, editors. Overview of vitamin D. Washington, DC: The National Academies Press;2011. p. 75–124.50. Arsenault JE, Mora-Plazas M, Forero Y, Lopez-Arana S, Marin C, Baylin A, et al. Provision of a school snack is associated with vitamin B-12 status, linear growth, and morbidity in children from Bogota, Colombia. J Nutr. 2009; 139:1744–1750. PMID: 19587125.
Article51. Parent AS, Teilmann G, Juul A, Skakkebaek NE, Toppari J, Bourguignon JP. The timing of normal puberty and the age limits of sexual precocity: variations around the world, secular trends, and changes after migration. Endocr Rev. 2003; 24:668–693. PMID: 14570750.
Article52. Danker-Hopfe H. Menarcheal age in Europe. Yearb Phys Anthropol. 1986; 29(Suppl 7):81–112.
Article53. Treloar SA, Martin NG. Age at menarche as a fitness trait: nonadditive genetic variance detected in a large twin sample. Am J Hum Genet. 1990; 47:137–148. PMID: 2349942.54. Yoshizawa T, Handa Y, Uematsu Y, Takeda S, Sekine K, Yoshihara Y, et al. Mice lacking the vitamin D receptor exhibit impaired bone formation, uterine hypoplasia and growth retardation after weaning. Nat Genet. 1997; 16:391–396. PMID: 9241280.
Article55. Kitagawa I, Kitagawa Y, Kawase Y, Nagaya T, Tokudome S. Advanced onset of menarche and higher bone mineral density depending on vitamin D receptor gene polymorphism. Eur J Endocrinol. 1998; 139:522–527. PMID: 9849817.
Article57. Lehtonen-Veromaa MK, Mottonen TT, Nuotio IO, Irjala KM, Leino AE, Viikari JS. Vitamin D and attainment of peak bone mass among peripubertal Finnish girls: a 3-y prospective study. Am J Clin Nutr. 2002; 76:1446–1453. PMID: 12450915.
Article58. Suarez F, Zeghoud F, Rossignol C, Walrant O, Garabedian M. Association between vitamin D receptor gene polymorphism and sex-dependent growth during the first two years of life. J Clin Endocrinol Metab. 1997; 82:2966–2970. PMID: 9284728.
Article59. El-Hajj Fuleihan G, Nabulsi M, Tamim H, Maalouf J, Salamoun M, Khalife H, et al. Effect of vitamin D replacement on musculoskeletal parameters in school children: a randomized controlled trial. J Clin Endocrinol Metab. 2006; 91:405–412. PMID: 16278262.
Article60. Deluca HF, Cantorna MT. Vitamin D: its role and uses in immunology. FASEB J. 2001; 15:2579–2585. PMID: 11726533.61. Sadeghi K, Wessner B, Laggner U, Ploder M, Tamandl D, Friedl J, et al. Vitamin D3 down-regulates monocyte TLR expression and triggers hyporesponsiveness to pathogen-associated molecular patterns. Eur J Immunol. 2006; 36:361–370. PMID: 16402404.
Article62. Piemonti L, Monti P, Sironi M, Fraticelli P, Leone BE, Dal Cin E, et al. Vitamin D3 affects differentiation, maturation, and function of human monocyte-derived dendritic cells. J Immunol. 2000; 164:4443–4451. PMID: 10779743.
Article63. Chiu KC, Chu A, Go VL, Saad MF. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr. 2004; 79:820–825. PMID: 15113720.
Article64. Timms PM, Mannan N, Hitman GA, Noonan K, Mills PG, Syndercombe-Court D, et al. Circulating MMP9, vitamin D and variation in the TIMP-1 response with VDR genotype: mechanisms for inflammatory damage in chronic disorders? QJM. 2002; 95:787–796. PMID: 12454321.
Article65. Rausch-Fan X, Leutmezer F, Willheim M, Spittler A, Bohle B, Ebner C, et al. Regulation of cytokine production in human peripheral blood mononuclear cells and allergen-specific th cell clones by 1alpha,25-dihydroxyvitamin D3. Int Arch Allergy Immunol. 2002; 128:33–41. PMID: 12037399.
Article66. Gregori S, Giarratana N, Smiroldo S, Uskokovic M, Adorini L. A 1alpha,25-dihydroxyvitamin D(3) analog enhances regulatory T-cells and arrests autoimmune diabetes in NOD mice. Diabetes. 2002; 51:1367–1374. PMID: 11978632.
Article67. Hypponen E, Laara E, Reunanen A, Jarvelin MR, Virtanen SM. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet. 2001; 358:1500–1503. PMID: 11705562.
Article68. Hypponen E, Sovio U, Wjst M, Patel S, Pekkanen J, Hartikainen AL, et al. Infant vitamin d supplementation and allergic conditions in adulthood: northern Finland birth cohort 1966. Ann N Y Acad Sci. 2004; 1037:84–95. PMID: 15699498.
Article69. Muhe L, Lulseged S, Mason KE, Simoes EA. Case-control study of the role of nutritional rickets in the risk of developing pneumonia in Ethiopian children. Lancet. 1997; 349:1801–1804. PMID: 9269215.
Article70. Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006; 311:1770–1773. PMID: 16497887.
Article71. Wayse V, Yousafzai A, Mogale K, Filteau S. Association of subclinical vitamin D deficiency with severe acute lower respiratory infection in Indian children under 5 y. Eur J Clin Nutr. 2004; 58:563–567. PMID: 15042122.
Article72. Roth DE, Jones AB, Prosser C, Robinson JL, Vohra S. Vitamin D receptor polymorphisms and the risk of acute lower respiratory tract infection in early childhood. J Infect Dis. 2008; 197:676–680. PMID: 18266602.
Article73. Banerji A, Bell A, Mills EL, McDonald J, Subbarao K, Stark G, et al. Lower respiratory tract infections in Inuit infants on Baffin Island. CMAJ. 2001; 164:1847–1850. PMID: 11450280.74. Hollis BW, Wagner CL. Assessment of dietary vitamin D requirements during pregnancy and lactation. Am J Clin Nutr. 2004; 79:717–726. PMID: 15113709.75. Hollis BW. Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: implications for establishing a new effective dietary intake recommendation for vitamin D. J Nutr. 2005; 135:317–322. PMID: 15671234.
Article76. Demay MB, Sabbagh Y, Carpenter TO. Calcium and vitamin D: what is known about the effects on growing bone. Pediatrics. 2007; 119(Suppl 2):S141–S144. PMID: 17332234.
Article77. Misra M, Pacaud D, Petryk A, Collett-Solberg PF, Kappy M. Drug and Therapeutics Committee of the Lawson Wilkins Pediatric Endocrine Society. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics. 2008; 122:398–417. PMID: 18676559.
Article78. Holick MF. Vitamin D deficienc y. N Engl J Med. 2007; 357:266–281. PMID: 17634462.79. Narchi H, El Jamil M, Kulaylat N. Symptomatic rickets in adolescence. Arch Dis Child. 2001; 84:501–503. PMID: 11369569.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Research advances in children's sleep and vitamin D levels
- Serum vitamin D status in children and adolescence with diabetes according to season and age
- Vitamin D in children with atopic dermatitis
- Whole blood and Plasma Vitamin C Concentrations of Elementary School Children in Chinju
- Recent Updates on Vitamin D and Pediatric Gastrointestinal Diseases