Korean Circ J.
2012 May;42(5):295-301. 10.4070/kcj.2012.42.5.295.
Mechanisms of Platelet Activation and Integrin alphaIIbeta3
- Affiliations
-
- 1Cardiology Division, Department of Internal Medicine, Jeju National University Hospital, Jeju, Korea. sejjoo@jejunu.ac.kr
- KMID: 2028756
- DOI: http://doi.org/10.4070/kcj.2012.42.5.295
Abstract
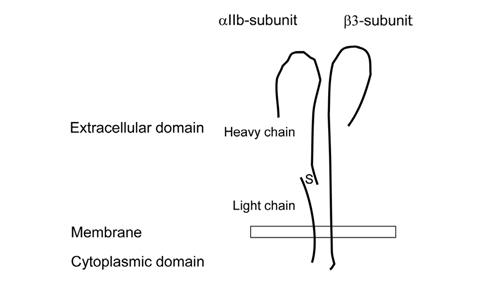
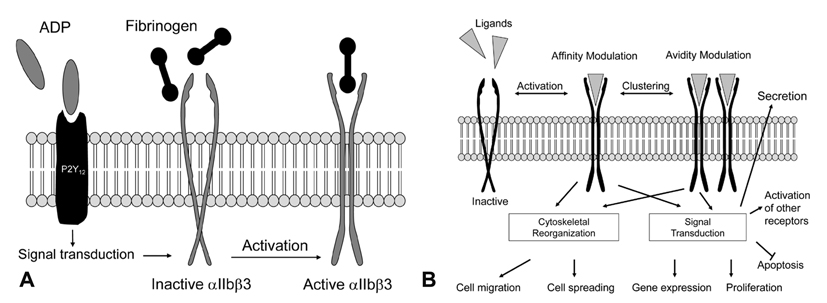
- Platelet aggregation is not only an essential part of hemostasis, but also initiates acute coronary syndrome or ischemic stroke. The precise understanding of the activation mechanism of platelet aggregation is fundamental for the development of more effective agents against platelet aggregation. Adenosine diphosphate, thrombin, and thromboxane A2 activate platelet integrin alphaIIbbeta3 through G protein-coupled receptors. G protein-mediated signaling pathways, which are initiated by Gq, G12/G13 or Gi, include phospholipase C with calcium signaling, Rho signaling, protein kinase C and phosphatidylinositol 3-kinase. Rap1b, Ca2+ and diacylglycerol-regulated guanine nucleotide exchange factor I, Rap1-GTP-interacting adaptor molecule, and Akt are important proteins involved in G protein-mediated activation of integrin alphaIIbbeta3. Binding of talin-1 and kindlin-3 to cytoplasmic domains of beta3-integrin triggers a conformational change in the extracellular domains that increases its affinity for ligands, such as fibrinogen or von Willebrand factor. Fibrinogens act as bridges between adjacent platelets to generate a platelet aggregate.
MeSH Terms
-
Acute Coronary Syndrome
Adenosine Diphosphate
Blood Platelets
Calcium Signaling
Cytoplasm
Fibrinogen
Guanine Nucleotide Exchange Factors
Hemostasis
Ligands
Phosphatidylinositol 3-Kinase
Platelet Activation
Platelet Aggregation
Platelet Glycoprotein GPIIb-IIIa Complex
Protein Kinase C
Proteins
Receptors, G-Protein-Coupled
Stroke
Thrombin
Thromboxane A2
Type C Phospholipases
von Willebrand Factor
Adenosine Diphosphate
Fibrinogen
Guanine Nucleotide Exchange Factors
Ligands
Phosphatidylinositol 3-Kinase
Platelet Glycoprotein GPIIb-IIIa Complex
Protein Kinase C
Proteins
Receptors, G-Protein-Coupled
Thrombin
Thromboxane A2
Type C Phospholipases
von Willebrand Factor
Figure
Cited by 1 articles
-
The New Weapon to Inhibit Proliferation and Migration of Smooth Muscle Cell in Neointimal Formation
Cheong-Whan Chae, Yoo-Wook Kwon
Korean Circ J. 2020;50(7):625-627. doi: 10.4070/kcj.2020.0197.
Reference
-
1. Joo SJ, Lee JW, Park YS. Increased activation of platelet glycoprotein IIb/IIIa in hypercholesterolemic patients. Korean Circ J. 1998. 28:2030–2041.2. Ruggeri ZM. Platelets in atherothrombosis. Nat Med. 2002. 8:1227–1234.3. Di Virgilio F, Solini A. P2 receptors: new potential players in atherosclerosis. Br J Pharmacol. 2002. 135:831–842.4. Dorsam RT, Kunapuli SP. Central role of the P2Y12 receptor in platelet activation. J Clin Invest. 2004. 113:340–345.5. Gachet C, Léon C, Hechler B. The platelet P2 receptors in arterial thrombosis. Blood Cells Mol Dis. 2006. 36:223–227.6. Oury C, Toth-Zsamboki E, Vermylen J, Hoylaerts MF. The platelet ATP and ADP receptors. Curr Pharm Des. 2006. 12:859–875.7. Nguyen TA, Diodati JG, Pharand C. Resistance to clopidogrel: a review of the evidence. J Am Coll Cardiol. 2005. 45:1157–1164.8. Brass S. Cardiovascular biology: platelets and proteases. Nature. 2001. 413:26–27.9. Offermanns S. Activation of platelet function through G protein-coupled receptors. Circ Res. 2006. 99:1293–1304.10. Smyth SS, Woulfe DS, Weitz JI, et al. G-protein-coupled receptors as signaling targets for antiplatelet therapy. Arterioscler Thromb Vasc Biol. 2009. 29:449–457.11. Becker RC, Moliterno DJ, Jennings LK, et al. Safety and tolerability of SCH 530348 in patients undergoing non-urgent percutaneous coronary intervention: a randomised, double-blind, placebo-controlled phase II study. Lancet. 2009. 373:919–928.12. Tricoci P, Huang Z, Held C, et al. Thrombin-receptor antagonist vorapaxar in acute coronary syndromes. N Engl J Med. 2012. 366:20–33.13. Giannarelli C, Zafar MU, Badimon JJ. Prostanoid and TP-receptors in atherothrombosis: is there a role for their antagonism? Thromb Haemost. 2010. 104:949–954.14. Offermanns S, Laugwitz KL, Spicher K, Schultz G. G proteins of the G12 family are activated via thromboxane A2 and thrombin receptors in human platelets. Proc Natl Acad Sci U S A. 1994. 91:504–508.15. Lefkovits J, Plow EF, Topol EJ. Platelet glycoprotein IIb/IIIa receptors in cardiovascular medicine. N Engl J Med. 1995. 332:1553–1559.16. Nieswandt B, Varga-Szabo D, Elvers M. Integrins in platelet activation. J Thromb Haemost. 2009. 7:Suppl 1. 206–209.17. Topol EJ, Byzova TV, Plow EF. Platelet GPIIb-IIIa blockers. Lancet. 1999. 353:227–231.18. Quinn MJ, Byzova TV, Qin J, Topol EJ, Plow EF. Integrin alphaIIbbeta3 and its antagonism. Arterioscler Thromb Vasc Biol. 2003. 23:945–952.19. Banno A, Ginsberg MH. Integrin activation. Biochem Soc Trans. 2008. 36(Pt 2):229–234.20. Chrzanowska-Wodnicka M, Smyth SS, Schoenwaelder SM, Fischer TH, White GC 2nd. Rap1b is required for normal platelet function and hemostasis in mice. J Clin Invest. 2005. 115:680–687.21. Cifuni SM, Wagner DD, Bergmeier W. CalDAG-GEFI and protein kinase C represent alternative pathways leading to activation of integrin alphaIIbbeta3 in platelets. Blood. 2008. 112:1696–1703.22. Crittenden JR, Bergmeier W, Zhang Y, et al. CalDAG-GEFI integrates signaling for platelet aggregation and thrombus formation. Nat Med. 2004. 10:982–986.23. Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003. 4:517–529.24. Varga-Szabo D, Braun A, Kleinschnitz C, et al. The calcium sensor STIM1 is an essential mediator of arterial thrombosis and ischemic brain infarction. J Exp Med. 2008. 205:1583–1591.25. Braun A, Varga-Szabo D, Kleinschnitz C, et al. Orai1 (CRACM1) is the platelet SOC channel and essential for pathological thrombus formation. Blood. 2009. 113:2056–2063.26. Lafuente EM, van Puijenbroek AA, Krause M, et al. RIAM, an Ena/VASP and Profilin ligand, interacts with Rap1-GTP and mediates Rap1-induced adhesion. Dev Cell. 2004. 7:585–595.27. Lee HS, Lim CJ, Puzon-McLaughlin W, Shattil SJ, Ginsberg MH. RIAM activates integrins by linking talin to ras GTPase membrane-targeting sequences. J Biol Chem. 2009. 284:5119–5127.28. Watanabe N, Bodin L, Pandey M, et al. Mechanisms and consequences of agonist-induced talin recruitment to platelet integrin alphaIIbbeta3. J Cell Biol. 2008. 181:1211–1222.29. Han J, Lim CJ, Watanabe N, et al. Reconstructing and deconstructing agonist-induced activation of integrin alphaIIbbeta3. Curr Biol. 2006. 16:1796–1806.30. Woulfe D, Jiang H, Morgans A, Monks R, Birnbaum M, Brass LF. Defects in secretion, aggregation, and thrombus formation in platelets from mice lacking Akt2. J Clin Invest. 2004. 113:441–450.31. Chen J, De S, Damron DS, Chen WS, Hay N, Byzova TV. Impaired platelet responses to thrombin and collagen in AKT-1-deficient mice. Blood. 2004. 104:1703–1710.32. Li D, August S, Woulfe DS. GSK3beta is a negative regulator of platelet function and thrombosis. Blood. 2008. 111:3522–3530.33. Stojanovic A, Marjanovic JA, Brovkovych VM, et al. A phosphoinositide 3-kinase-AKT-nitric oxide-cGMP signaling pathway in stimulating platelet secretion and aggregation. J Biol Chem. 2006. 281:16333–16339.34. Zhang W, Colman RW. Thrombin regulates intracellular cyclic AMP concentration in human platelets through phosphorylation/activation of phosphodiesterase 3A. Blood. 2007. 110:1475–1482.35. Bennett JS, Berger BW, Billings PC. The structure and function of platelet integrins. J Thromb Haemost. 2009. 7:Suppl 1. 200–205.36. Critchley DR, Gingras AR. Talin at a glance. J Cell Sci. 2008. 121(Pt 9):1345–1347.37. Li R, Babu CR, Valentine K, et al. Characterization of the monomeric form of the transmembrane and cytoplasmic domains of the integrin beta 3 subunit by NMR spectroscopy. Biochemistry. 2002. 41:15618–15624.38. Lau TL, Partridge AW, Ginsberg MH, Ulmer TS. Structure of the integrin beta3 transmembrane segment in phospholipid bicelles and detergent micelles. Biochemistry. 2008. 47:4008–4016.39. Petrich BG, Marchese P, Ruggeri ZM, et al. Talin is required for integrin-mediated platelet function in hemostasis and thrombosis. J Exp Med. 2007. 204:3103–3111.40. Nieswandt B, Moser M, Pleines I, et al. Loss of talin1 in platelets abrogates integrin activation, platelet aggregation, and thrombus formation in vitro and in vivo. J Exp Med. 2007. 204:3113–3118.41. Larjava H, Plow EF, Wu C. Kindlins: essential regulators of integrin signalling and cell-matrix adhesion. EMBO Rep. 2008. 9:1203–1208.42. Moser M, Nieswandt B, Ussar S, Pozgajova M, Fässler R. Kindlin-3 is essential for integrin activation and platelet aggregation. Nat Med. 2008. 14:325–330.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Flow Cytometric Detection of Platelet Activation in Preeclampsia Compared with Uncomplicated Pregnancy
- CD98 activation increases surface expression and clusteringof beta 1 integrins in MCF-7 cells through FAK/Src- and cytoskeleton-independent mechanisms
- Analysis of platelet glycoprotein IIIa by flow cytometry and diagnosis of Glanzmann's thrombasthenia
- Effects of Long Term Hormone Therapy on Platelet Activation in Postmenopausal Women
- Observation on the platelet activation in disseminated intravascular coagulation