J Korean Diabetes Assoc.
2007 Jan;31(1):22-32. 10.4093/jkda.2007.31.1.22.
Oxidative Stress Causes Vascular Insulin Resistance in OLETF Rat Through Increased IRS-1 Degradation
- Affiliations
-
- 1Department of Internal Medicine, School of Medicine, Pusan National University, Korea.
- 2Department of Internal Medicine, College of Medicine1, Dongguk University, Korea.
- KMID: 2008093
- DOI: http://doi.org/10.4093/jkda.2007.31.1.22
Abstract
-
BACKGROUND: Insulin resistance and oxidative stress have been reported to play essential pathophysiological roles in diabetic cardiovascular complication. The relationship between insulin resistance and oxidative stress in vasculature remains unclear. The study was conducted to assess whether oxidative stress induce vascular insulin resistance in OLETF rat, a model of type 2 diabetes
METHODS
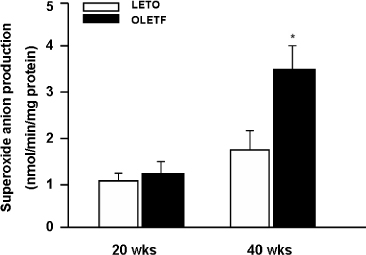
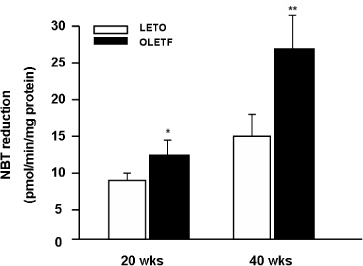
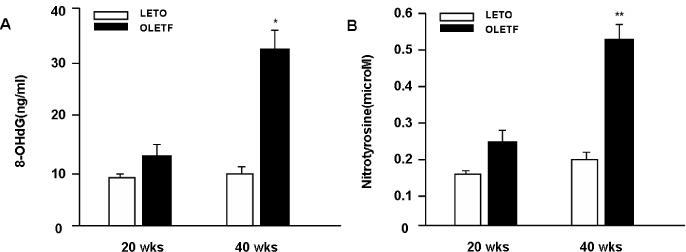
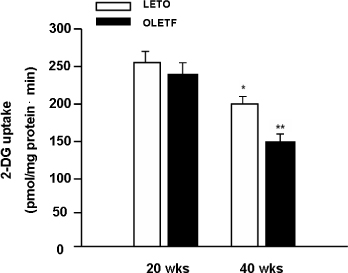
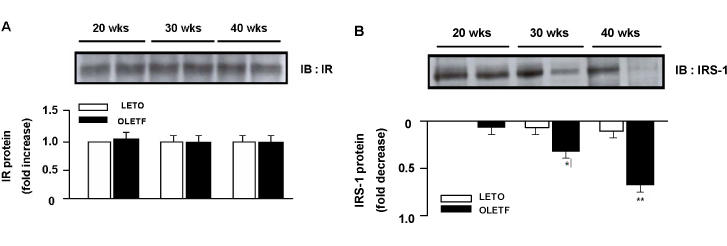
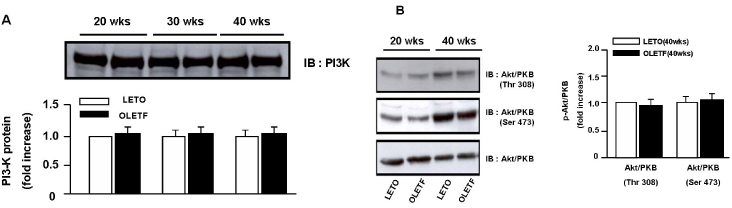
We used OLETF rats (20/30/40 weeks, n = 5/5/5), as models of type 2 DM, and LETO rats (20/30/40 weeks, n = 5/5/5) as controls. Aortas of each rats were extracted. Superoxide anion production was detected by NBT assay and lucigenin assay. 8-hydroxyguanosine (OHdG) and nitrotyrosine were detected as markers of oxidative stress in 20 and 40 weeks groups. The glucose uptake of aortas was measured by detecting 2-deoxyglucose uptake in both groups. The expression of IR, IRS-1, PI3-K and Akt/PKB were detected by immuno precipitation and immunoblotting in 20, 30 and 40 weeks groups
RESULTS
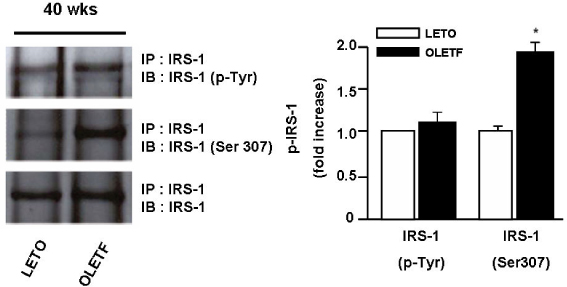
Superoxide anion production and markers of oxidative stress (8-OHdG, nitrotyrosine) were significantly increased in aortas of OLETF rats compared with controls. Aortas of OLETF rats exhibited decreased IRS-1 content and increased phosphorylation of IRS-1 at Ser307 compared with LETO rats. There were no significant differences in expressions of IR, PI3-K and Akt/PKB between two groups
CONCLUSION
These results suggest that oxidative stress induces insulin resistance in vasculature of OLETF rat specifically through increasing serine phosphorylation of IRS-1 and its degradation by a proteasome-dependent pathway, providing an alternative mechanism that may explain the association with insulin resistance and diabetic vascular complications.
Keyword
MeSH Terms
Figure
Reference
-
1. Steiner C. Diabetes and atherosclerosis. Diabetes. 1981. 30:Suppl 2. S1–S7.2. The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the developmentand progression of long-term complication in insulin-dependent diabetes mellitus. N Eng J Med. 1993. 329:977–986.3. Nourooz-Zadeh J, Tauaddini-Surmadi J, McCarthy S, Betteridge DJ, Wolff SP. Elevated levels of authentic plasma hydroperoxides in NIDDM. Diabetes. 1995. 44:1054–1058.4. Frustaci A, et al. Myocardial cell death in human diabetes. Circ Res. 2000. 87:1123–1132.5. Hunt JV, Dean RT, Wolff SP. Hydroxy radical production and and autoxidative glycosylation: glucose autoxidation as the cause of protein damage in the experimental glycation model of diabetes mellitus and aging. Biochem J. 1988. 256:205–212.6. Schmidt AM, Hori O, Brett J, Yan SD, Wautler JL, Stern D. Cellular receptors for advanced glycation end products: implications for induction of oxidant stress and cellular dysfunction in the pathogenesis of vascular lesions. Arterioscle Thromb Vasc Biol. 1994. 14:1521–1528.7. Matsubara T, Ziff M. Increased superoxide anion release from human endothelial cells in response to cytokines. J Immunol. 1986. 137:3295–3298.8. Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Cir Res. 1994. 74:1141–1148.9. Pagano PJ, Chanock SJ, Siwik DA, Colucci WS, Clark JK. Angiotensin II induces p67phox mRNA expression and NADPH oxidase superoxide generation in rabbit aortic adventitial fibroblasts. Hypertension. 1998. 32:331–337.10. Sen CK, Packer L. Antioxidant and redox regulation of gene transcription. FASEB J. 1996. 10:709–720.11. Li PF, Dietz R, von Harsdorf R. Reactive oxygen species induce apoptosis of vascular smooth muscle cell. FEBS Lett. 1997. 404:249–252.12. Maron BJ, Nishimura RA, McKenna WJ, Rakowski J, Josephson ME, Kieval RS. Assessment of permanent dual-chamber pacing as a treatment for drug-refractory symptomatic patients with obstructive hypertrophic cardiomyopathy. Circulation. 1999. 99:2934–2941.13. Westhuyzen J. The oxidation hypothesis of atherosclerosis: An update. Ann Clin Lab Sci. 1997. 27:1–10.14. Ginsberg HN. Insulin resistance and cardiovascular disease. J Clin Invest. 2000. 106:453–458.15. Freener EP, King GL. Vascular dysfunction in diabetic mellitus. Lancet. 1997. 350:Suppl 1. SI9–SI13.16. Paolisso G, Giuglianoc D. Oxidative stress and insulin action: Is there a relationship? Diabetologia. 1996. 39:357–363.17. Gardner CD, Eguchi S, Reynolds CM, Eguchi K, Frank GD, Motley ED. Hydrogen peroxide inhibits insulin signaling in vascular smooth muscle cells. Exp Biol Med. 2003. 228:836–842.18. Ohara Y, Peterson TX, Sayegh HS, Subramanian RR, Wilcox JN, Harrison DG. Dietary correlation of hypercholesterolemia in the rabbit normalizes endothelial superoxide anion production. Circulation. 1995. 92:898–903.19. Ohara Y, Peterson TE, Harrison DG. Hypercholesterolemia increases endothelial superoxide anion production. J Clin Invest. 1993. 91:2546–2551.20. Pourcyrous M, Leffler CW, Bada HS, Korones SB, Busija DW. Brain superoxide anion generation in asphyxiated piglets and the effect of indomethacin at therapeutic dose. Pediatr Res. 1993. 34:366–369.21. Yan SD, Schmidt AM, Anderson GM, Zhang J, Brett J, Zou YS, Pinsky D, Stern D. Enhanced celluar oxidant stress by the interaction of advanced glycation end products with their receptors/binding proteins. J Biol Chem. 1994. 269:9889–9897.22. Son SM, Whalin MK, Harrison DG, Taylor WR, Griendling KK. Oxidative stress and diabetic vascular complications. Curr Diab Rep. 2004. 4:247–252.23. Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M, Aoki T, Etoh T, Hashimoto T, Naruse M, Sano H, Utsumi H, Nawata H. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C-dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes. 2000. 49:1939–1945.24. Zou MH, Shi C, Cohen RA. High glucose via peroxynitrite causes tyrosine nitration and inactivation of prostacyclin synthase that is associated with TP receptor-mediated apoptosis and adhesion molecule expression in cultured human aortic endothelial cells. Diabetes. 2002. 51:198–203.25. Tannous M, Rabini RA, Vignini A, Moretti N, Fumelli P, Zielinski B, Mazzanti L, Mutus B. Evidence for iNOS-dependent peroxynitrite production in diabetic platelets. Diabetologia. 1999. 42:539–544.26. Ceriello A, Quagliro L, D'Amico M, Di Filippo C, Marfella R, Nappo F, Berrino L, Rossi F, Giugliano D. Acute hyperglycemia induces nitrotyrosine formation and apoptosis in perfused heart from rat. Diabetes. 2002. 51:1076–1082.27. Dandona P, Thusu K, Cook S, Snyder B, Makowski J, Armstrong D, Nicotera T. Oxidative damage to DNA in diabetes mellitus. Lancet. 1996. 347:444–445.28. Kerr SM, Brosnan MJ, MeIntyre M, Reid JL, Dominiczak AF, Hamilton CA. Superoxide anion production is increased in a model of genetic hypertension: the role of the endothelium. Hypertension. 1999. 33:1353–1358.29. Miller FJ, Gutterman DD, Rios CD, Heistad DD, Davidson BL. Superoxide production in vascular smooth muscle contributes to oxidative stress and impaired relaxation in atherosclerosis. Circ Res. 1998. 82:1298–1305.30. Wang HD, Pagano PJ, Du Y, Cayatte AJ, Quinn MT, Brecher P, Cohen RA. Superoxide anion from the adventitia of the rat thoracic aorta inactivates nitric oxide. Circ Res. 1998. 82:810–818.31. Cheatham B, Kahn CR. Insulin action and the insulin signaling network. Endocr Rev. 1995. 16:117–142.32. Chen R, Kim O, Yang J. Regulation of Akt/PKB activation by tyrosine phosphorylation. J Biol Chem. 2001. 276:31858–31862.33. Sykiotis GP, Papavassiliou AG. Serine phosphorylation of insulin receptor substrate-1; a novel target for the reversal of insulin resistance. Mol Endocrinol. 2001. 15:1864–1869.34. Ventre J, Doebber T, Wu M. Targeted disruption of the tumor necrosis factor-alpha gene: metabolic consequences in obese and nonobese mice. Diabetes. 1997. 46:1526–1531.35. Greene MW, Sakaue H, Wang L, Alessi DR, Roth RA. Modulation of insulin-stimulated degradation of human insulin receptor substrate-1 by Serine 312 phosphorylation. J Biol Chem. 2003. 278:8199–8211.36. Hirata AE, Alvarez-Rojas F, Carvalheira JB, Carvalho CR, Dolnikoff MS, Abdalla Saad MJ. Modulation of IR/PTP1B interaction and downstream signaling in insulin sensitive tissues of MSG-rats. Life Sci. 2003. 73:1369–1381.37. De Fea K, Roth RA. Protein kinase C modulation of insulin receptor substrate-1 tyrosine phosphorylation requires serine 612. Biochemistry. 1997. 36:12939–12947.38. Aguirre V, Werner ED, Giraud J, Lee YH, Shoelson SE, White MF. Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J Biol Chem. 2002. 277:1531–1537.39. Pederson TM, Kramer DL, Rondinone CM. Serine/threonine phosphorylation of IRS-1 triggers its degradation: possible regulation by tyrosine phosphorylation. Diabetes. 2001. 50:24–31.40. Greene MW, Sakaue H, Wang L, Alessi DR, Roth RA. Modulation of insulin-stimulated degradation of human insulin receptor substrate-1 by Serine 312 phosphorylation. J Biol Chem. 2003. 278:8199–8211.41. Kaiser N, Sasson S, Freener EP, Boukobza-Vardi N, Higashi S, Moller DE, Davidheiser S, Przybylski RJ, King GL. Differential regulation of glucose transport and transporters by glucose in vascular endothelial and smooth muscle cells. Diabetes. 1993. 42:80–89.42. Banz WJ, Abel MA, Zemel MB. Insulin regulation of vascular smooth muscle glucose transport in insulin-sensitive and resistant rats. Horm Metab Res. 1996. 28:271–275.43. Gjugliano D, Ceriello A, Paolisso G. Oxidative stress and diabetic vascular complications. Diabetes Care. 1996. 19:257–267.44. Cai H, Harrison DG. Endothelial dysfunction in cardiovascular disease: the role of oxidant stress. Circ Res. 2000. 87:840–844.45. Ceriello A. Oxidative stress and glycemic regulation. Metabolism. 2000. 49:27–29.46. Taniyama Y, Hitomi H, Shah A, Alexander W, Griendling KK. Mechanism of reactive oxygen species-dependent downregulation of insulin receptor substrate-1 by angiotensin II. Arterioscler Thromb Vasc Biol. 2005. 25:1142–1147.47. Seshiah PN, Weber DS, Rocic P, Valppu L, Taniyama Y, Griendling KK. Angiotensin II stimulation of NAD(P)H oxidase activity: upstream mediators. Circ Res. 2002. 91:406–413.48. Taniyama Y, Weber DS, Rocic P, Hilenski L, Akers ML, Park J, Hemmings BA, Alexander RW, Griendling KK. Pyk2- and Src-dependent tyrosine phosphorylation of PDK1 regulates focal adhesions. Mol Cell Biol. 2003. 23:8019–8029.49. Tokor A, Newton AC. Cellular signaling: pivoting around PDK-1. Cell. 2000. 103:185–188.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The long-term effect of ramipril on Gialpha2-protein and Protein Tyrosine Phosphatase 1B in an animal model of type 2 diabetes(OLETF rat)
- O-GlcNAc modification on IRS-1 and Akt2 by PUGNAc inhibits their phosphorylation and induces insulin resistance in rat primary adipocytes
- Can antioxidants be effective therapeutics for type 2 diabetes?
- Pathogenesis and Management of Non-alcoholic Steatohepatitis
- Patterns of Insulin Resistance Syndrome in the Taegu Community for the Development of Nutritional Service Improvement Programs