J Korean Acad Conserv Dent.
2006 Sep;31(5):398-408. 10.5395/JKACD.2006.31.5.398.
Evaluation of periodontal ligament cell viability in rat teeth according to various extra-oral dry storage times using MTT assay
- Affiliations
-
- 1Department of Conservative Dentistry, College of Dentistry, Yonsei University, Seoul, Korea. sjlee@yumc.yonsei.ac.kr
- 2Oral Cancer Research Institute, College of Dentistry, Yonsei University, Seoul, Korea.
- 3Oral Science Research Center, College of Dentistry, Yonsei University, Seoul, Korea.
- KMID: 1986883
- DOI: http://doi.org/10.5395/JKACD.2006.31.5.398
Abstract
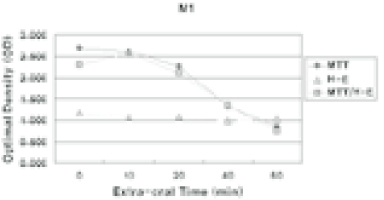
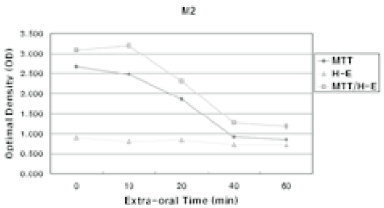
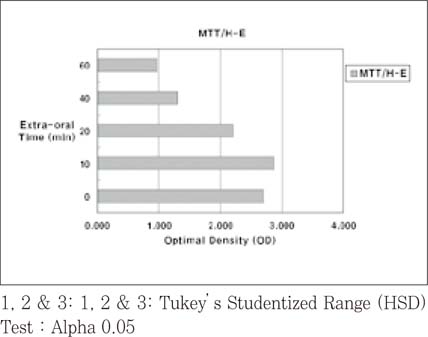
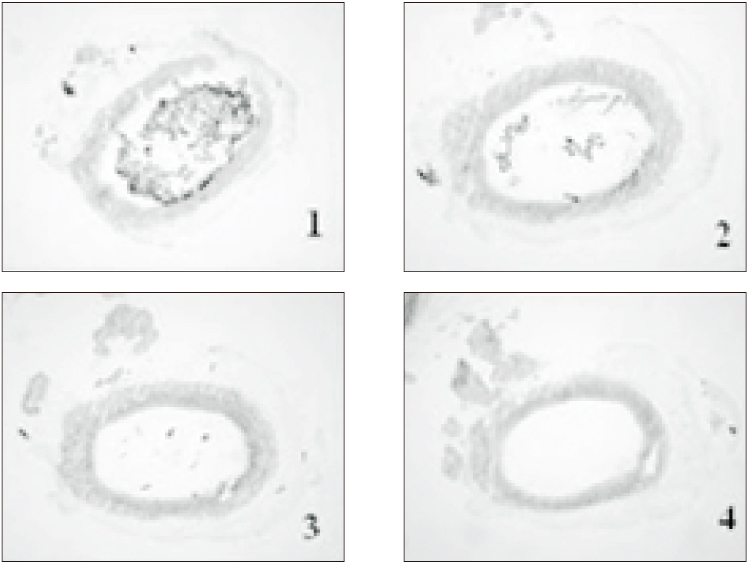
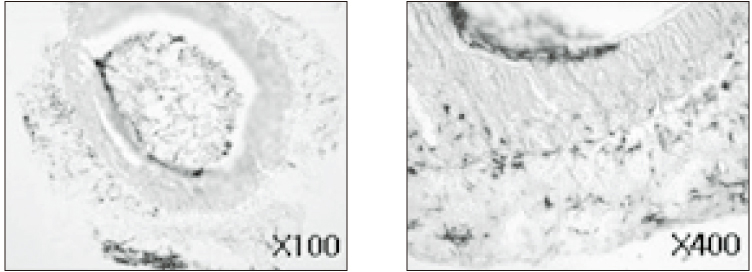
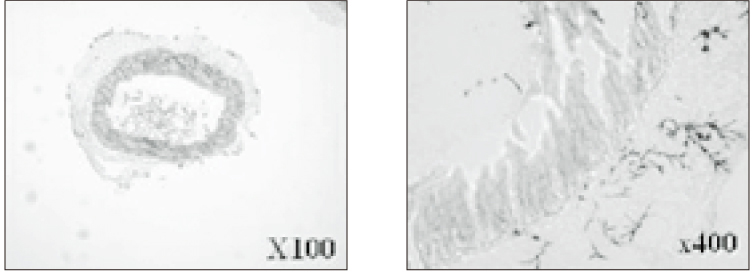
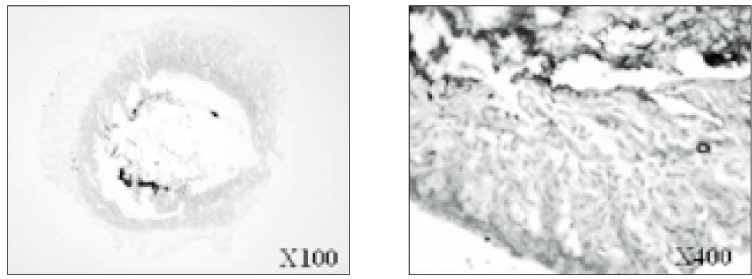
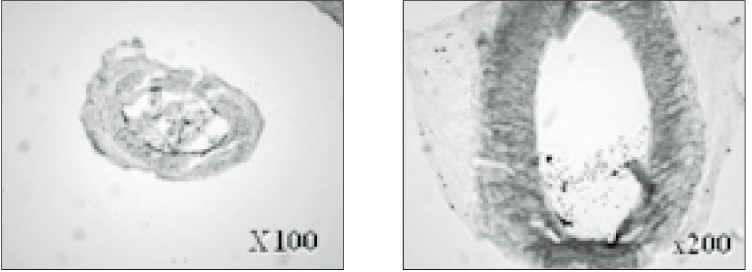
- The purpose of this study was to verify the usefulness of MTT analysis as a tool of measurement of the periodontal ligament cell viability from the extracted rat molar. A total of 80 Sprague-Dawley white female rat of 4 week-old with a body weight of 100 grams were used. The maxillary left and right, first and second molars were extracted under Ketamine anesthesia. Twenty-four teeth of each group (divided as five groups depending upon the time-lapse after extraction such as immediate, 10, 20, 40 and 60 minutes) were immersed in 200 microl of MTT solution (0.5 mg/ml) and processed for optical density measurements . Another 10 teeth of each group were treated as same as above and sectioned at 10 microm for microscopic examination. All measurements values were divided by the value of hematoxylin-eosin staining which represented the volume of each corresponding samples. Immediate and 10 minute groups showed highest MTT values followed by 20, 40, and 60 minutes consecutively. Statistical significance (p < 0.05) existed between all groups except in immediate versus 10 minute groups and 40 versus 60 minutes. Histological findings also showed similar findings with MTT results in crystal shape and crystal numbers between the experimental groups. These data indicate that in vivo MTT analysis may be of value for evaluation of the periodontal ligament cell viability without time- consuming cell culturing processes.
MeSH Terms
Figure
Reference
-
1. Lee SJ, Jung IY, Lee CY, Choi SY, Kum KY. Clinical application of computer-aided rapid prototyping for tooth tranaplantation. Dent Traumatol. 2001. 17:114–119.
Article2. Andreasen JO, Kristerson L. The effect of limited drying or removal of the periodontal ligament. Periodontal healing after replantation of mature permanent incisors in monkeys. Acta Odontol Scand. 1981. 39:1–13.3. Patel S, Dumsha TC, Sydiskis RJ. Determining periodontal ligament cell vitality from exarticulated teeth stored in saline or milk using fluorescein diacetate. Int Endod J. 1994. 27:1–5.
Article4. Hupp JG, Trope M, Mesaros SV, Aukhil I. Tritiated thymidine uptake in periodontal ligament cells of dogs' teeth stored in various media for extended time periods. Endod Dent Traumatol. 1997. 13:223–227.
Article5. Lekic P, Kenny D, Barrett E. implications for tooth replantation. Int Endod J. 1998. 31:137–140.6. Ashkenazi M, Sarnat H, Keila S. In vitro viability, mitogenicity and clonogenic capacity of periodontal ligament cells after storage in six different media. Endod Dent Traumatol. 1999. 15(4):149–156.
Article7. Mosmann T. Rapid colorimetric assay for cellular growth and survival : application to proliferation and cytotoxicity assays. J Immunol Methods. 1983. 65(1-2):55–63.
Article8. Kim HK, Kim ES, Choi IB, Kim J, Lee SJ. The verification of the MTT assay on the viability of periodontal ligamental cells in rat molars through the histologic examination. J Korean Acad Conserv Dent. 2003. 28(5):385–391.
Article9. Bordin S, Page RC, Narayanan AS. isolation and characterization of one phenotype. Science. 1984. 223:171–173.10. Narayanan AS, Page RC. Connective tissues of the periodontium : a summary of current work. Coll Relat Res. 1983. 3:33–64.11. McCulloch CAG, Knowles G. Discrimination of two fibroblast progenitor population in early explant cultures of hamster gingiva. Cell Tissue Res. 1991. 264:87–94.
Article12. Bornstein P. The cross-linking of collagen and elastin and its inhibition in osteolathyrism. Is there a relation to the aging process? Am J Med. 1970. 49:429–435.
Article13. Barrington EP, Meyer J. Recovery of the rat dental organ from experimental lathyrism. J Periodontol. 1966. 37:453–467.
Article14. Lindskog S, Blomlöf L. Influence of osmolarity and composition of some storage media on human periodontal ligament cells. Acta Odontol Scand. 1982. 40:435–441.15. Andersson L, Bodin I. Avulsed human teeth replanted within 15 minutes- a long-term clinical follow-up study. Endod Dent Traumatol. 1990. 6:37–42.
Article16. Schour J, Massler M. Farris EJ, Griffith JQ, editors. The teeth. The Rat in Laboratory Investigation. 1949. 2nd edn. Philadelphia: J.B. Lippincott.17. Modeer T, Dahllof , Otteskog P. Effect of drying on human periodontal ligament repair in vitro. J Int Assoc Dent Child. 1984. 15:15–20.18. Soder PO, Otteskog P, Andreasen JO, Modeer T. Effect of drying on viability of the periodontal membrane. Scand J Dent Res. 1977. 85:164–168.19. Lekic P, Kenny D, Moe HK, Barrett E, McCulloch CA. Relationship of clonogenic capacity to plating efficiency and vital dye staining of human periodontal ligament cells; implications for tooth replantation. J Periodontal Res. 1996. 31:294–300.
Article20. Cho MI, Garant PR. The effect of beta-aminoproprionitrile on the periodontal ligament: I. Ultrastructure of fibroblasts and matrix. J Periodontal Res. 1984. 19:247–260.
Article21. Cho MI, Garant PR. The effect of beta-aminoproprionitrile on the periodontal ligament: II. Radioautographic study of collagen secretion from fibroblasts. Anat Rec. 1984. 209:41–52.
Article22. Cho MI, Matsuda N, Lin WL, Moshier A, Ramakrishnan PR. In vitro formation of mineralized nodules by periodontal ligament cells from the rat. Calcif Tissue Int. 1992. 50:459–467.
Article23. McCulloch CA. Basic considerations in periodontal wound healing to achieve regeneration. Periodontol 2000. 1993. 1:16–25.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation of the Viability of Rat Periodontal Ligament Cells after Storing at 0℃/2 MPa Condition up to One Week: In Vivo MTT Method
- Evaluation of the viability of periodontal ligament cell in rat teeth using slow cryopreservation method with magnetic field
- The efficacy of programmed cryo-preservation under pressure in rat periodontal ligament cells
- The evaluation of periodontal ligament cells of rat teeth after low-temperature preservation under high pressure
- Evaluation of periodontal ligament cell viability in rat teeth after frozen preservation using in-vivo MTT assay