Korean J Orthod.
2008 Jun;38(3):187-201. 10.4041/kjod.2008.38.3.187.
The expression of MMP-1, -8, and -13 mRNA in the periodontal ligament of rats during tooth movement with cortical punching
- Affiliations
-
- 1Department of Orthodontics, School of Dentistry, Pusan National University, Korea. softid@pusan.ac.kr
- 2Department of Oral & Maxillofacial Surgery, School of Dentistry, Pusan National University, Korea.
- 3Medical Science Research Institute, Pusan National University Hospital, Korea.
- KMID: 1975907
- DOI: http://doi.org/10.4041/kjod.2008.38.3.187
Abstract
OBJECTIVE
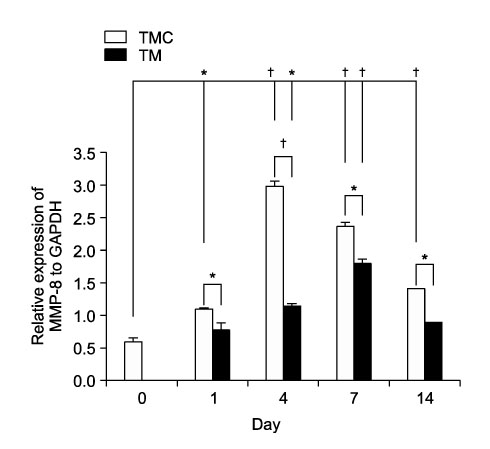
The aim of this study was to determine whether cortical punching stimulates the expression of matrix metalloproteinase-1, -8, and -13 in orthodontic tooth movement in rats. METHODS: A total of 32 male sprague-dawley rats at 15 weeks old were divided into two groups of 16 rats each, to form the tooth movement with cortical punching (TMC) group and tooth movement only (TM) group. A total of 20 gm of orthodontic force was applied to rat incisors to cause experimental tooth movement. Cortical punching was performed on the palatal side near the central incisor with a 1.0 mm width microscrew in the TMC group. The duration of tooth movement was 1, 4, 7, and 14 days. RESULTS: Measurements of the mRNA expression were selected as the means to determine the identification of expression of MMP-1, -8, and -13. In the TMC group, the expression of collagen type I was greater than that of the TM group from day 4 to day 14. Expression of TIMP-1 in the TM group was greater than that of the TMC group in the pressure side of PDL and alveolar bone cell at day 4. In the TMC group, TIMP-1 was expressed at the osteoclast, but not at the tooth surface of the TM group at day 14. Maximum induction of the mRNA of MMP-1 was observed on day 4 in the TMC group, but it was observed on day 7 in the TM group. MMP-8 mRNA of the TMC group was twice greater than that of the TM group at 4 days. In the TMC group, maximum induction of MMP-13 mRNA was observed on day 1. CONCLUSIONS: These findings suggested that cortical punching can stimulate remodeling of PDL and alveolar bone connective tissues during experimental orthodontic tooth movement in rats.
MeSH Terms
-
Animals
Collagen Type I
Connective Tissue
Humans
Incisor
Male
Matrix Metalloproteinase 1
Osteoclasts
Periodontal Ligament
Rats
Rats, Sprague-Dawley
RNA, Messenger
Tissue Inhibitor of Metalloproteinase-1
Tooth
Tooth Movement
Collagen Type I
Matrix Metalloproteinase 1
RNA, Messenger
Tissue Inhibitor of Metalloproteinase-1
Figure
Cited by 2 articles
-
Observation of trabecular changes of the mandible after orthognathic surgery using fractal analysis
Hyeon-Ju Kang, Song-Wha Jeong, Bong-Hye Jo, Yong-Deok Kim, Seong Sik Kim
J Korean Assoc Oral Maxillofac Surg. 2012;38(2):96-100. doi: 10.5125/jkaoms.2012.38.2.96.Histologic assessment of the biological effects after speedy surgical orthodontics in a beagle animal model: a preliminary study
Hong-Suk Kim, Young-Jun Lee, Young-Guk Park, Kyu-Rhim Chung, Yoon-Goo Kang, HyeRan Choo, Seong-Hun Kim
Korean J Orthod. 2011;41(5):361-370. doi: 10.4041/kjod.2011.41.5.361.
Reference
-
1. Yamasaki K, Shibata Y, Fukuhara T. The effect of prostaglandins on experimental tooth movement in monkeys (Macaca fuscata). J Dent Res. 1982. 61:1444–1446.
Article2. Takano-Yamamoto T, Kawakami M, Kobayasi Y, Yamashiro T, Sakuda M. The effect of local application of 1,25-dihydroxycholecalciferol on osteoclast numbers in orthodontically treated rats. J Dent Res. 1992. 71:53–59.
Article3. Soma S, Yamashita K, Matsumoto S, Takada K. Effect of continuous infusion of PTH on orthodontic tooth movement. J Jpn Orthod Soc Abstr. 1997.4. Frost HM. The regional accelerated phenomenon. Orthop Clin North Am. 1981. 12:725–726.5. Frost HM. The regional acceleratory phenomenon: a review. Henry Ford Hosp Med J. 1983. 31:3–9.6. Frost HM. Skeletal structural adaptations to mechanical usage (SATMU): 2. Redefining Wolffs law: the remodeling problem. Anat Rec. 1990. 226:414–422.
Article7. Kole H. Surgical operations on the alveolar ridge to correct occlusal abnormalities. Oral Surg Oral Med Oral Pathol. 1959. 12:515–529.
Article8. Wilcko WM, Wilcko T, Bouquot JE, Ferguson DJ. Rapid orthodontics with alveolar reshaping: two case reports of decrowding. Int J Periodontics Restorative Dent. 2001. 21:9–19.9. Kwan Tat S, Padrines M, Théoleyre S, Heymann D, Fortun Y. IL-6, RANKL, TNF-alpha/IL-1: interrelations in bone resorption pathophysiology. Cytokine Growth Factor Rev. 2004. 15:49–60.
Article10. Lauer-Fields JL, Juska D, Fields GB. Matrix metalloproteinases and collagen catabolism. Biopolymers. 2002. 66:19–32.
Article11. Vu TH, Werb Z. Matrix metalloproteinases: effectors of development and normal physiology. Genes Dev. 2000. 14:2123–2133.
Article12. Tsubota M, Sasano Y, Takahashi I, Kagayama M, Shimauchi H. Expression of MMP-8 and MMP-13 mRNAs in rat periodontium during tooth eruption. J Dent Res. 2002. 81:673–678.
Article13. Ren Y, Maltha JC, Kuijpers-jagtman AM. The rat as a model for orthodontic tooth movement-a critical review and proposed solution. Eur J Orthod. 2004. 26:483–490.
Article14. Pfeifer JS. The reaction of alveolar bone to flap procedures in man. Periodontics. 1965. 3:135–140.15. Anholm JM, Crites DA, Hoff R, Rathbun WE. Calif Dent Assoc. 1986. 14:7–11.16. Gantes B, Rathbun E, Anholm M. Effects on the periodontium following corticotomy-facilitated orthodontics. Case reports. J Periodontol. 1990. 61:234–238.
Article17. Kana JS, Hutschenreiter G, Haina D, Waidelich W. Effect of low-power density laser radiation on healing of open skin wounds in rats. Arch Surg. 1981. 116:293–296.
Article18. Mester E, Mester AF, Mester A. The biomedical effects of laser application. Lasers Surg Med. 1985. 5:31–39.
Article19. Bosatra M, Jucci A, Olliaro P, Quacci D, Sacchi S. In vitro fibroblast and dermis fibroblast activation by laser irradiation at low energy. An electron microscopic study. Dermatologica. 1984. 168:157–162.
Article20. Lam TS, Abergel RP, Meeker CA, Castel JC, Dwyer RM, Uitto J. Laser stimulation of collagen synthesis in human skin fibroblast cultures. Lasers Life Sci. 1986. 1:61–77.21. Rygh P. Norton LA, Burstone CJ, editors. The biology of tooth movement. 1989. Boca Raton, FL: CRC Press;9–28.22. Igarashi K, Miyoshi K, Shinoda H, Saeki S, Mitani H. Diurnal variation in tooth movement in response to orthodontic force in rats. Am J Orthod Dentofacial Orthop. 1998. 114:8–14.
Article23. Kanzaki H, Chiba M, Shimizu Y, Mitani H. Dual regulation of osteoclast differentiation by periodontal ligament cells through RANKL stimulation and OPG inhibition. J Dent Res. 2001. 80:887–891.
Article24. Kanzaki H, Chiba M, Shimizu Y, Mitani H. Periodontal ligament cells under mechanical stress induce osteoclastogenesis by receptor activator of nuclear factor kappaB ligand up-regulation via prostaglandin E2 synthesis. J Bone Miner Res. 2002. 17:210–220.
Article25. Domon S, Shimokawa H, Matsumoto Y, Yamaguchi S, Soma K. In situ hybridization for matrix metalloproteinase-1 and cathepsin K in rat root-resorbing tissue induced by tooth movement. Arch Oral Biol. 1999. 44:907–915.
Article26. Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, et al. Matrix metalloproteinases: a review. Critic Rev Oral Biol Med. 1993. 4:197–250.
Article27. Beertsen W, Everts V. The site of remodelling of collagen in the periodontal ligament of the mouse incisor. Anat Rec. 1977. 189:479–497.
Article28. Beertsen W, Brekelmans M, Everts V. The site of collagen resorption in the periodontal ligament of the rodent molar. Anat Rec. 1978. 192:305–317.
Article29. Circolo A, Welgus HG, Pierce GF, Kramer J, Strunk RC. Differential regulation of the expression of proteinases/antiproteinases in fibroblasts. Effects of interleukin-1 and platelet-derived growth factor. J Biol Chem. 1991. 266:12283–12288.
Article30. Salonen J, Uitto VJ, Pan YM, Oda D. Proliferating oral epithelial cells in culture are capable of both extracellular and intracellular degradation of interstitial collagen. Matrix. 1991. 11:43–55.
Article31. Berg RA, SchwarTz ML, Crystal RG. Regulation of the production of secretory proteins: intracellular degradation of newly synthesized "defective" collagen. Proc NatL Acad Sci USA. 1980. 77:4746–4750.
Article32. Melcher AH, Chan J. Phagocytosis and digestion of collagen by gingival fibroblasts in vivo: a study of serial sections. J Ultrastruct Res. 1981. 77:1–36.
Article33. Everts V, Wolvius E, Saklatvala J, Beertsen W. Interleukin 1 increases the production of collagenase but does not influence the phagocytosis of collagen fibrils. Matrix. 1990. 10:388–393.
Article34. Gronowicz G, Hadjimichael J, Richards D, Cerami A, Rossomando EF. Correlation between tumor necrosis factor-( TNF-alpha)-induced cytoskeletal changes and human collagenase gene induction. J Periodontal Res. 1992. 27:562–568.
Article35. Sodek J, Ferrier JM. Collagen remodelling in rat periodontal tissues: compensation for precursor reutilization confirms rapid turnover of collagen. Coll Relat Res. 1988. 8:11–21.
Article36. Werb Z, Hembry RM, Murphy G, Aggeler J. Commitment to expression of the metalloendopeptidases, collagenase and stromelysin: relationship of inducing events to changes in cytoskeletal architecture. J Cell Biol. 1986. 102:697–702.
Article37. Parakkal PF. Involvement of macrophages in collagen resorption. J Cell Biol. 1969. 41:345–354.
Article38. Ten Cate AR, Freeman E. Collagen remodelling by fibroblasts in wound repair. Preliminary observations. Anat Rec. 1974. 179:543–546.
Article39. Sorsa T, Uitto VJ, Suomalainen K, Vauhkonen M, Lindy S. Comparison of interstitial collagenase from human gingiva, sulcular fluid and polymorphonuclear leukocytes. J Periodontal Res. 1988. 23:386–393.
Article40. Ingman T, Sorsa T, Suomalainen K, Halinen S, Lindy O, Lauhio A, et al. Tetracycline inhibition and the cellular source of collagenase in gingival crevicular fluid in different periodontal diseases. A review article. J Periodontol. 1993. 64:82–88.
Article41. Kryshtalskyj E, Sodek J, Ferrier JM. Correlation of collagenolytic enzymes and inhibitors in gingival crevicular fluid with clinical and microscopic changes in experimental periodontitis in the dog. Arch Oral Biol. 1986. 31:21–31.
Article42. Kryshtalskyj E, Sodek J. Nature of collagenolytic enzyme and inhibitor activities in crevicular fluid from healthy and inflamed periodontal tissues of beagle dogs. J Periodontal Res. 1987. 22:264–269.
Article43. Takahashi I, Nishimura M, Onodera K, Bae JW, Mitani H, Okazaki M, et al. Expression of MMP-8 and MMP-13 genes in the periodontal ligament during tooth movement in rats. J Dent Res. 2003. 82:646–651.
Article44. Iozzo RV. Matrix proteoglycans: from molecular design to cellular function. Annu Rev Biochem. 1998. 67:609–652.
Article45. Harris ED, Cartwright EC. Barrett AJ. Mammalian collagenases. Proteinases in mammalian cells and tissues. 1997. Amsterdam: Elsevier/North Holland Biomedical Press;249–283.46. Harris ED Jr, Krane SM. Collagenases (second of three parts). N Engl J Med. 1974. 291:605–609.47. van der Zee E, Everts V, Beertsen W. Cytokines modulate contraction of periosteal explants from rabbit calvariae. Connect Tissue Res. 1995. 31:141–151.
Article48. van der Zee E, Everts V, Beertsen W. Cytokines modulate routes of collagen breakdown. Review with special emphasis on mechanisms of collagen degradation in the periodontium and the burst hypothesis of periodontal disease progression. J Clin Periodontol. 1997. 24:297–305.49. Redlich M, Reichenberg E, Harari D, Zaks B, Shoshan S, Palmon A. The effect of mechanical force on mRNA levels of collagenase, collagen type I, and tissue inhibitors of metalloproteinases in gingiva of dogs. J Dent Res. 2001. 80:2080–2084.
Article50. Holliday LS, Vakani A, Archer L, Dolce C. Effects of matrix metalloproteinase inhibitors on bone resorption and orthodontic tooth movement. J Dent Res. 2003. 82:687–691.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The effect of cortical punching on the expression of OPG, RANK, and RANKL in the periodontal tissue during tooth movement in rats
- A study on the expression of vascular endothelial growth factor in tension side of rat molar periodontal ligament following experimental tooth movement
- The expression of heat shock protein in the experimental tooth movement in rats
- Inhibiton of MMP-13 mRNA expression by Doxycycline combination with Mefenamic Acid in the rat Periodontal ligament cells
- Role of MAP kinase on MMP-13 expression in rat periodontal ligament cells