J Periodontal Implant Sci.
2014 Apr;44(2):79-84. 10.5051/jpis.2014.44.2.79.
A periodontitis-associated multispecies model of an oral biofilm
- Affiliations
-
- 1Department of Periodontology, Research Institute of Oral Sciences, Gangneung-Wonju National University College of Dentistry, Gangneung, Korea. dentist@gwnu.ac.kr
- 2Department of Microbiology and Immunology, Research Institute of Oral Sciences, Gangneung-Wonju National University College of Dentistry, Gangneung, Korea.
- KMID: 1914508
- DOI: http://doi.org/10.5051/jpis.2014.44.2.79
Abstract
- PURPOSE
While single-species biofilms have been studied extensively, we know notably little regarding multispecies biofilms and their interactions. The purpose of this study was to develop and evaluate an in vitro multispecies dental biofilm model that aimed to mimic the environment of chronic periodontitis.
METHODS
Streptococcus gordonii KN1, Fusobacterium nucleatum ATCC23726, Aggregatibacter actinomycetemcomitans ATCC33384, and Porphyromonas gingivalis ATCC33277 were used for this experiment. The biofilms were grown on 12-well plates with a round glass slip (12 mm in diameter) with a supply of fresh medium. Four different single-species biofilms and multispecies biofilms with the four bacterial strains listed above were prepared. The biofilms were examined with a confocal laser scanning microscope (CLSM) and scanning electron microscopy (SEM). The minimum inhibitory concentrations (MIC) for four different planktonic single-species and multispecies bacteria were determined. The MICs of doxycycline and chlorhexidine for four different single-species biofilms and a multispecies biofilm were also determined.
RESULTS
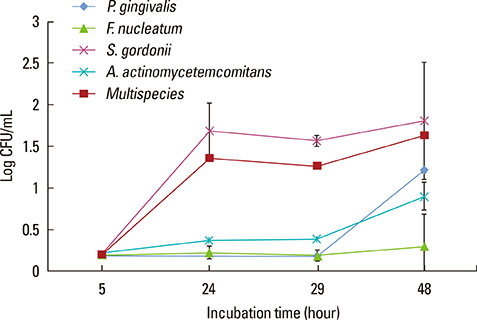
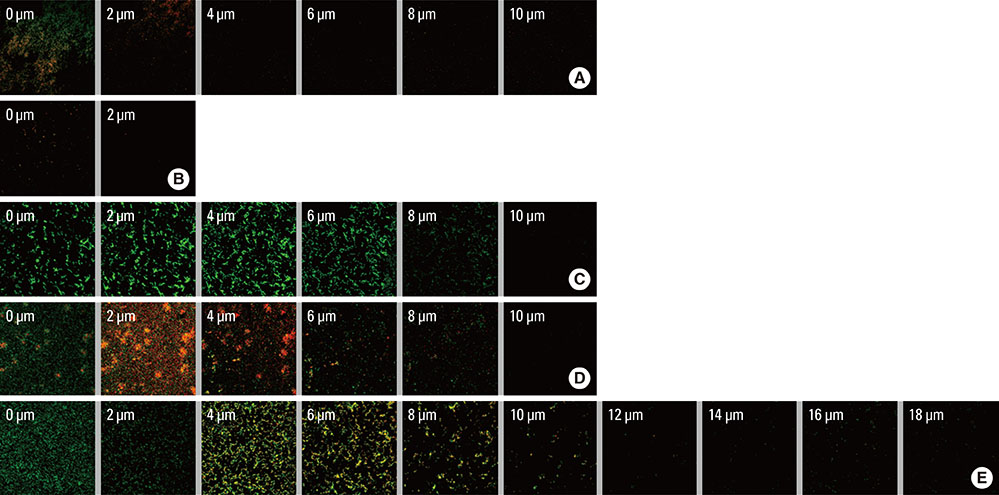
The CLSM and SEM examination revealed that the growth pattern of the multispecies biofilm was similar to those of single-species biofilms. However, the multispecies biofilm became thicker than the single-species biofilms, and networks between bacteria were formed. The MICs of doxycycline and chlorhexidine were higher in the biofilm state than in the planktonic bacteria. The MIC of doxycycline for the multispecies biofilm was higher than were those for the single-species biofilms of P. gingivalis, F. nucleatum, or A. actinomycetemcomitans. The MIC of chlorhexidine for the multispecies biofilm was higher than were those for the single-species biofilms of P. gingivalis or F. nucleatum.
CONCLUSIONS
To mimic the natural dental biofilm, a multispecies biofilm composed of four bacterial species was grown. The 24-hour multispecies biofilm may be useful as a laboratory dental biofilm model system.
MeSH Terms
Figure
Cited by 1 articles
-
Comparison of periodontitis-associated oral biofilm formation under dynamic and static conditions
Won sub Song, Jae-Kwan Lee, Se Hwan Park, Heung-Sik Um, Si Young Lee, Beom-Seok Chang
J Periodontal Implant Sci. 2017;47(4):219-230. doi: 10.5051/jpis.2017.47.4.219.
Reference
-
1. Socransky SS, Haffajee AD. Dental biofilms: difficult therapeutic targets. Periodontol 2000. 2002; 28:12–55.
Article2. Holt SC, Ebersole JL. Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia: the "red complex", a prototype polybacterial pathogenic consortium in periodontitis. Periodontol 2000. 2005; 38:72–122.
Article3. Kolenbrander PE, Palmer RJ Jr, Periasamy S, Jakubovics NS. Oral multispecies biofilm development and the key role of cell-cell distance. Nat Rev Microbiol. 2010; 8:471–480.
Article4. James GA, Korber DR, Caldwell DE, Costerton JW. Digital image analysis of growth and starvation responses of a surface-colonizing Acinetobacter sp. J Bacteriol. 1995; 177:907–915.
Article5. Kolenbrander PE, Palmer RJ Jr, Rickard AH, Jakubovics NS, Chalmers NI, Diaz PI. Bacterial interactions and successions during plaque development. Periodontol 2000. 2006; 42:47–79.
Article6. Fux CA, Costerton JW, Stewart PS, Stoodley P. Survival strategies of infectious biofilms. Trends Microbiol. 2005; 13:34–40.
Article7. Wilson M. Susceptibility of oral bacterial biofilms to antimicrobial agents. J Med Microbiol. 1996; 44:79–87.
Article8. Sissons CH. Artificial dental plaque biofilm model systems. Adv Dent Res. 1997; 11:110–126.
Article9. Schlafer S, Raarup MK, Meyer RL, Sutherland DS, Dige I, Nyengaard JR, et al. pH landscapes in a novel five-species model of early dental biofilm. PLoS One. 2011; 6:e25299.
Article10. Kolenbrander PE, Andersen RN, Blehert DS, Egland PG, Foster JS, Palmer RJ Jr. Communication among oral bacteria. Microbiol Mol Biol Rev. 2002; 66:486–505.
Article11. Sader HS, Flamm RK, Jones RN. Antimicrobial activity of daptomycin tested against Gram-positive pathogens collected in Europe, Latin America, and selected countries in the Asia-Pacific Region (2011). Diagn Microbiol Infect Dis. 2013; 75:417–422.
Article12. Ximenez-Fyvie LA, Haffajee AD, Socransky SS. Comparison of the microbiota of supra- and subgingival plaque in health and periodontitis. J Clin Periodontol. 2000; 27:648–657.
Article13. Prates RA, Yamada AM Jr, Suzuki LC, Eiko Hashimoto MC, Cai S, Gouw-Soares S, et al. Bactericidal effect of malachite green and red laser on Actinobacillus actinomycetemcomitans. J Photochem Photobiol B. 2007; 86:70–76.
Article14. Wright TL, Ellen RP, Lacroix JM, Sinnadurai S, Mittelman MW. Effects of metronidazole on Porphyromonas gingivalis biofilms. J Periodontal Res. 1997; 32:473–477.
Article15. Sanchez MC, Llama-Palacios A, Blanc V, Leon R, Herrera D, Sanz M. Structure, viability and bacterial kinetics of an in vitro biofilm model using six bacteria from the subgingival microbiota. J Periodontal Res. 2011; 46:252–260.
Article16. Periasamy S, Kolenbrander PE. Mutualistic biofilm communities develop with Porphyromonas gingivalis and initial, early, and late colonizers of enamel. J Bacteriol. 2009; 191:6804–6811.
Article17. Walker C, Sedlacek MJ. An in vitro biofilm model of subgingival plaque. Oral Microbiol Immunol. 2007; 22:152–161.
Article18. Prescott JF, Baggot JD. Antimicrobial susceptibility testing and antimicrobial drug dosage. J Am Vet Med Assoc. 1985; 187:363–368.19. Song HH, Lee JK, Um HS, Chang BS, Lee SY, Lee MK. Phototoxic effect of blue light on the planktonic and biofilm state of anaerobic periodontal pathogens. J Periodontal Implant Sci. 2013; 43:72–78.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Quorum Sensing Regulation of Biofilm Formation by Periodontal Pathogens
- Comparison of Antimicrobial Activity of Electrolyzed Water Using Various Electrodes against Biofilm of Oral Pathogens
- Effect of Photothermal Therapy with Indocyanine Green in Multispecies Biofilm
- Endodontic biofilms: contemporary and future treatment options
- Inhibition of biofilm formation of periodontal pathogens by D-Arabinose