Anat Cell Biol.
2014 Jun;47(2):83-90. 10.5115/acb.2014.47.2.83.
Adipogenesis of Sprague Dawely rats mesenchymal stem cells: a morphological, immunophenotyping and gene expression follow-up study
- Affiliations
-
- 1Department of Zoology, Urology and Nephrology Center, Research Center, Mansoura University, Cairo, Egypt. mbam@mans.edu.eg
- KMID: 1882578
- DOI: http://doi.org/10.5115/acb.2014.47.2.83
Abstract
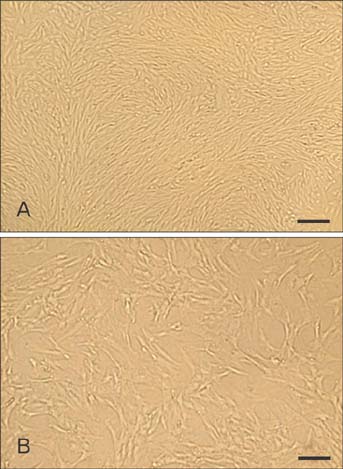
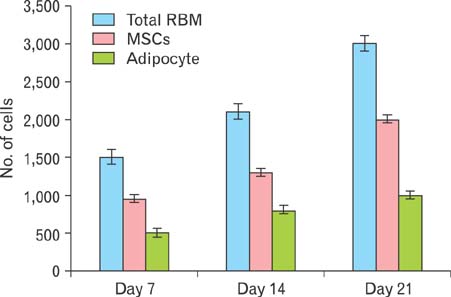
- Mesenchymal stem cells (MSCs) offer significant promise as a multipotent source for cell-based therapies and could form the basis for the differentiation and cultivation of tissue grafts to replace damaged tissue. However, no gene expression follow up analysis has been undertaken to characterize the in vitro adipogenic differentiated MSCs. The main goal of this study was to focus on MSCs and to analyze their differentiation capacity. To achieve this aim, bone marrow MSCs from sprague dawely rats were isolated, expanded in monolayer culture and characterized with respect to their cluster of differentiation (CD) and ability for adipogenic differentiation capacity. The expression of CD44, CD45, CD29, CD34, and CD90 on bone marrow derived MSCs was characterized using flow cytometry. Adipogenesis was determined by staining with oil-red O and reverse transcription polymerase chain reaction assessments of lipoprotein lipase, leptin, adiponectin and adipocyte genes at different time intervals, after 4, 7, 14, and 21 days. Our results revealed that the pattern of CD marker expression was highly positive significant with CD29, CD44, and CD90 when compared with CD34 and CD45. MSCs showed proliferative potential and were capable of adipogenic differentiation characterized by reddish brown-droplets following staining with oil-red O and expression of molecular bands of genes. These results demonstrate, at the morphological, immunophenotyping and gene expression levels, the multipotency of MSCs and thus highlight their potential therapeutic value for cell-based tissue engineering.
Keyword
MeSH Terms
Figure
Reference
-
1. Caplan AI, Bruder SP. Mesenchymal stem cells: building blocks for molecular medicine in the 21st century. Trends Mol Med. 2001; 7:259–264.2. Leo AJ, Grande DA. Mesenchymal stem cells in tissue engineering. Cells Tissues Organs. 2006; 183:112–122.3. Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006; 98:1076–1084.4. Till JE, McCulloch EA. Hemopoietic stem cell differentiation. Biochim Biophys Acta. 1980; 605:431–459.5. Wickham MQ, Erickson GR, Gimble JM, Vail TP, Guilak F. Multipotent stromal cells derived from the infrapatellar fat pad of the knee. Clin Orthop Relat Res. 2003; (412):196–212.6. Alexanian AR, Sieber-Blum M. Differentiating adult hippocampal stem cells into neural crest derivatives. Neuroscience. 2003; 118:1–5.7. Williams JT, Southerland SS, Souza J, Calcutt AF, Cartledge RG. Cells isolated from adult human skeletal muscle capable of differentiating into multiple mesodermal phenotypes. Am Surg. 1999; 65:22–26.8. Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991; 9:641–650.9. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999; 284:143–147.10. Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968; 6:230–247.11. Stockl S, Bauer RJ, Bosserhoff AK, Gottl C, Grifka J, Grassel S. Sox9 modulates cell survival and adipogenic differentiation of multipotent adult rat mesenchymal stem cells. J Cell Sci. 2013; 126:2890–2902.12. Mackay AM, Beck SC, Murphy JM, Barry FP, Chichester CO, Pittenger MF. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng. 1998; 4:415–428.13. Jaiswal N, Haynesworth SE, Caplan AI, Bruder SP. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem. 1997; 64:295–312.14. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006; 8:315–317.15. Kadiyala S, Young RG, Thiede MA, Bruder SP. Culture expanded canine mesenchymal stem cells possess osteochondrogenic potential in vivo and in vitro. Cell Transplant. 1997; 6:125–134.16. Lennon DP, Caplan AI. Isolation of rat marrow-derived mesenchymal stem cells. Exp Hematol. 2006; 34:1606–1607.17. Phinney DG, Kopen G, Isaacson RL, Prockop DJ. Plastic adherent stromal cells from the bone marrow of commonly used strains of inbred mice: variations in yield, growth, and differentiation. J Cell Biochem. 1999; 72:570–585.18. Green H, Meuth M. An established pre-adipose cell line and its differentiation in culture. Cell. 1974; 3:127–133.19. Hauner H, Schmid P, Pfeiffer EF. Glucocorticoids and insulin promote the differentiation of human adipocyte precursor cells into fat cells. J Clin Endocrinol Metab. 1987; 64:832–835.20. Peng HH, Wang TH, Chao AS, Chang SD. Isolation and differentiation of human mesenchymal stem cells obtained from second trimester amniotic fluid; experiments at Chang Gung Memorial Hospital. Chang Gung Med J. 2007; 30:402–407.21. Rubin J, Murphy TC, Fan X, Goldschmidt M, Taylor WR. Activation of extracellular signal-regulated kinase is involved in mechanical strain inhibition of RANKL expression in bone stromal cells. J Bone Miner Res. 2002; 17:1452–1460.22. Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001; 29:e45.23. de Macedo Braga LM, Lacchini S, Schaan BD, Rodrigues B, Rosa K, De Angelis K, Borges LF, Irigoyen MC, Nardi NB. In situ delivery of bone marrow cells and mesenchymal stem cells improves cardiovascular function in hypertensive rats submitted to myocardial infarction. J Biomed Sci. 2008; 15:365–374.24. Ayatollahi M, Salmani MK, Geramizadeh B, Tabei SZ, Soleimani M, Sanati MH. Conditions to improve expansion of human mesenchymal stem cells based on rat samples. World J Stem Cells. 2012; 4:1–8.25. Mageed AS, Pietryga DW, DeHeer DH, West RA. Isolation of large numbers of mesenchymal stem cells from the washings of bone marrow collection bags: characterization of fresh mesenchymal stem cells. Transplantation. 2007; 83:1019–1026.26. Mareddy S, Crawford R, Brooke G, Xiao Y. Clonal isolation and characterization of bone marrow stromal cells from patients with osteoarthritis. Tissue Eng. 2007; 13:819–829.27. Bayati V, Hashemitabar M, Gazor R, Nejatbakhsh R, Bijannejad D. Expression of surface markers and myogenic potential of rat bone marrow- and adipose-derived stem cells: a comparative study. Anat Cell Biol. 2013; 46:113–121.28. Jin HJ, Bae YK, Kim M, Kwon SJ, Jeon HB, Choi SJ, Kim SW, Yang YS, Oh W, Chang JW. Comparative analysis of human mesenchymal stem cells from bone marrow, adipose tissue, and umbilical cord blood as sources of cell therapy. Int J Mol Sci. 2013; 14:17986–18001.29. Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007; 100:1249–1260.30. Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006; 116:1784–1792.31. Zhu W, Cheng KK, Vanhoutte PM, Lam KS, Xu A. Vascular effects of adiponectin: molecular mechanisms and potential therapeutic intervention. Clin Sci (Lond). 2008; 114:361–374.32. Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999; 257:79–83.33. Torigoe M, Matsui H, Ogawa Y, Murakami H, Murakami R, Cheng XW, Numaguchi Y, Murohara T, Okumura K. Impact of the high-molecular-weight form of adiponectin on endothelial function in healthy young men. Clin Endocrinol (Oxf). 2007; 67:276–281.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Characterization of Mesenchymal Stem Cells Derived from Rat Bone Marrow and Adipose Tissue: A Comparative Study
- IL6 Receptor Facilitates Adipogenesis Differentiation of Human Mesenchymal Stem Cells through Activating P38 Pathway
- The Role of Beta-Tricalcium Phosphate-Hydrogel Scaffold and Mesenchymal Stem Cells on Neogenic Bone Formation
- The N- and C-terminal domains of parathyroid hormone-related protein affect differently the osteogenic and adipogenic potential of human mesenchymal stem cells
- Transdifferentiation from Adipogenic bone Marrow Derived Stromal Cell to Osteoblast