J Periodontal Implant Sci.
2015 Jun;45(3):120-126. 10.5051/jpis.2015.45.3.120.
Microgrooves on titanium surface affect peri-implant cell adhesion and soft tissue sealing; an in vitro and in vivo study
- Affiliations
-
- 1Department of Periodontology, Section of Dentistry, Seoul National University Bundang Hospital, Seongnam, Korea.
- 2Department of Oral Health Sciences, Medical University of South Carolina College of Dental Medicine, Charleston, SC, USA.
- 3Department of Periodontics, Dankook University College of Dentistry Jukjeon Dental Hospital, Yongin, Korea.
- 4Department of Oral Pathology, Seoul National University School of Dentistry, Seoul, Korea.
- 5Department of Dental Biomaterials, Seoul National University School of Dentistry, Seoul, Korea.
- 6Department of Public Health Sciences, Korea University, Seoul, Korea.
- 7Department of General Dentistry, Boston University School of Dental Medicine, Boston, MA, USA.
- 8Department of Periodontology, Seoul National University School of Dentistry, Seoul, Korea. periopf@snu.ac.kr
- 9Dental Research Institute, Seoul National University School of Dentistry, Seoul, Korea.
- KMID: 1824941
- DOI: http://doi.org/10.5051/jpis.2015.45.3.120
Abstract
- PURPOSE
With the significance of stable adhesion of alveolar bone and peri-implant soft tissue on the surface of titanium for successful dental implantation procedure, the purpose of this study was to apply microgrooves on the titanium surface and investigate their effects on peri-implant cells and tissues.
METHODS
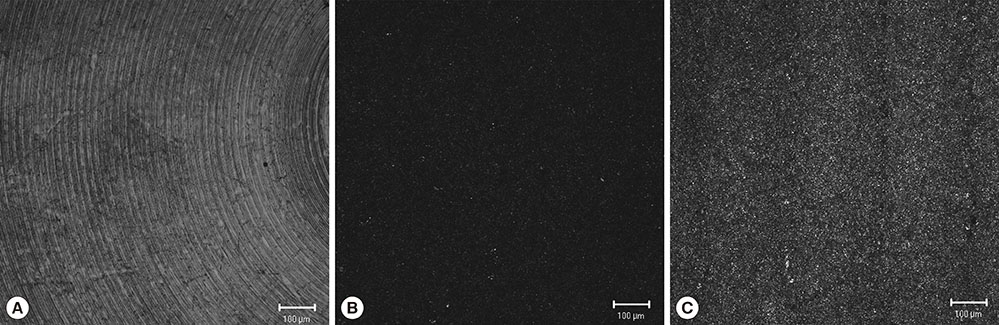
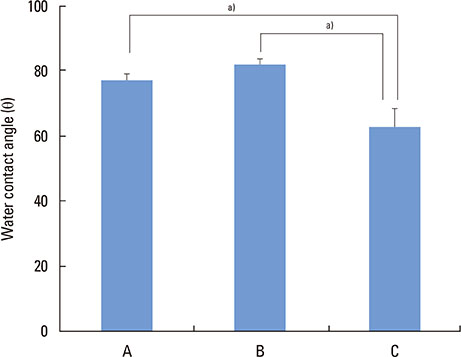
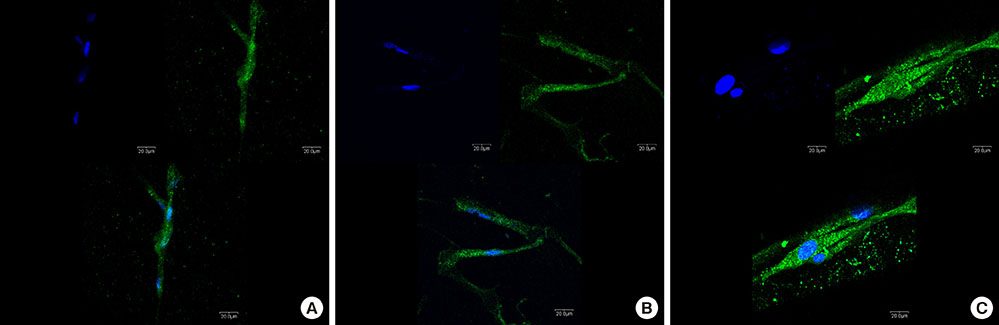
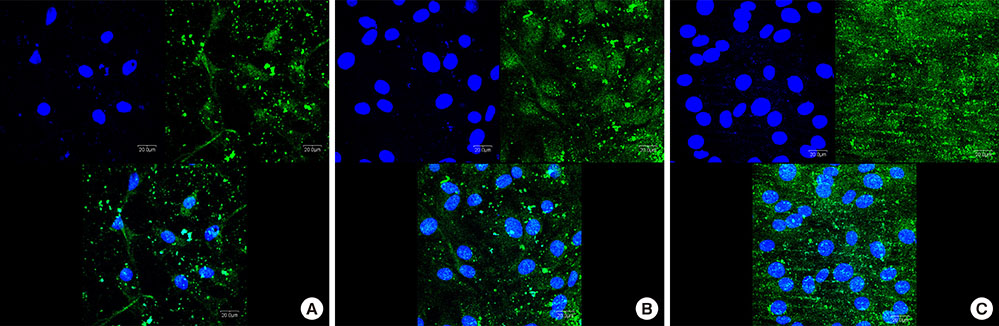
Three types of commercially pure titanium discs were prepared; machined-surface discs (A), sandblasted, large-grit, acid-etched (SLA)-treated discs (B), SLA and microgroove-formed discs (C). After surface topography of the discs was examined by confocal laser scanning electron microscopy, water contact angle and surface energy were measured. Human gingival fibroblasts (hGFs) and murine osteoblastic cells (MC3T3-E1) were seeded onto the titanium discs for immunofluorescence assay of adhesion proteins. Commercially pure titanium implants with microgrooves on the coronal microthreads design were inserted into the edentulous mandible of beagle dogs. After 2 weeks and 6 weeks of implant insertion, the animal subjects were euthanized to confirm peri-implant tissue healing pattern in histologic specimens.
RESULTS
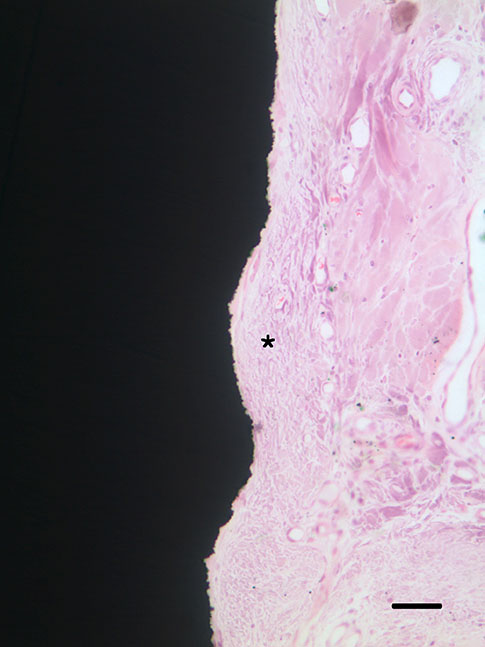
Group C presented the lowest water contact angle (62.89+/-5.66 theta), highest surface energy (45+/-1.2 mN/m), and highest surface roughness (Ra=22.351+/-2.766 microm). The expression of adhesion molecules of hGFs and MC3T30E1 cells was prominent in group C. Titanium implants with microgrooves on the coronal portion showed firm adhesion to peri-implant soft tissue.
CONCLUSIONS
Microgrooves on the titanium surface promoted the adhesion of gingival fibroblasts and osteoblastic cells, as well as favorable peri-implant soft tissue sealing.
Keyword
MeSH Terms
Figure
Reference
-
1. Brånemark PI, Adell R, Albrektsson T, Lekholm U, Lundkvist S, Rockler B. Osseointegrated titanium fixtures in the treatment of edentulousness. Biomaterials. 1983; 4:25–28.
Article2. Hermann JS, Buser D, Schenk RK, Higginbottom FL, Cochran DL. Biologic width around titanium implants. A physiologically formed and stable dimension over time. Clin Oral Implants Res. 2000; 11:1–11.
Article3. Geurs NC, Vassilopoulos PJ, Reddy MS. Soft tissue considerations in implant site development. Oral Maxillofac Surg Clin North Am. 2010; 22:387–405. vi–vii.
Article4. Abrahamsson I, Zitzmann NU, Berglundh T, Linder E, Wennerberg A, Lindhe J. The mucosal attachment to titanium implants with different surface characteristics: an experimental study in dogs. J Clin Periodontol. 2002; 29:448–455.
Article5. Huh JB, Rheu GB, Kim YS, Jeong CM, Lee JY, Shin SW. Influence of Implant transmucosal design on early peri-implant tissue responses in beagle dogs. Clin Oral Implants Res. 2014; 25:962–968.
Article6. Owens DK, Wendt RC. Estimation of surface free energy of polymers. J Appl Polym Sci. 1969; 13:1741–1747.7. Bächle M, Kohal RJ. A systematic review of the influence of different titanium surfaces on proliferation, differentiation and protein synthesis of osteoblast-like MG63 cells. Clin Oral Implants Res. 2004; 15:683–692.
Article8. Cooper LF, Zhou Y, Takebe J, Guo J, Abron A, Holmén A, et al. Fluoride modification effects on osteoblast behavior and bone formation at TiO2 grit-blasted c.p. titanium endosseous implants. Biomaterials. 2006; 27:926–936.
Article9. Kim MJ, Choi MU, Kim CW. Activation of phospholipase D1 by surface roughness of titanium in MG63 osteoblast-like cell. Biomaterials. 2006; 27:5502–5511.
Article10. Marinucci L, Balloni S, Becchetti E, Belcastro S, Guerra M, Calvitti M, et al. Effect of titanium surface roughness on human osteoblast proliferation and gene expression in vitro. Int J Oral Maxillofac Implants. 2006; 21:719–725.11. Sader MS, Balduino A, Soares GD, Borojevic R. Effect of three distinct treatments of titanium surface on osteoblast attachment, proliferation, and differentiation. Clin Oral Implants Res. 2005; 16:667–675.
Article12. Cochran DL, Hermann JS, Schenk RK, Higginbottom FL, Buser D. Biologic width around titanium implants. A histometric analysis of the implanto-gingival junction around unloaded and loaded nonsubmerged implants in the canine mandible. J Periodontol. 1997; 68:186–198.
Article13. Ruggeri A, Franchi M, Marini N, Trisi P, Piatelli A. Supracrestal circular collagen fiber network around osseointegrated nonsubmerged titanium implants. Clin Oral Implants Res. 1992; 3:169–175.
Article14. Broggini N, McManus LM, Hermann JS, Medina R, Schenk RK, Buser D, et al. Peri-implant inflammation defined by the implant-abutment interface. J Dent Res. 2006; 85:473–478.
Article15. Chehroudi B, Gould TR, Brunette DM. The role of connective tissue in inhibiting epithelial downgrowth on titanium-coated percutaneous implants. J Biomed Mater Res. 1992; 26:493–515.
Article16. Linkevicius T, Apse P. Biologic width around implants. An evidence-based review. Stomatologija. 2008; 10:27–35.17. Gargiulo AW, Wentz FM, Orban B. Dimensions and relations of dentogingival junction in humans. J Periodontol. 1961; 32:261–267.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of titanium surface microgrooves and thermal oxidation on in vitro osteoblast responses
- Chitosan/hydroxyapatite composite coatings on porous Ti6Al4V titanium implants: in vitro and in vivo studies
- Improvement of peri-implant complications through customized prosthesis restoration allowing soft tissue space: a case report
- Development of Zinc-Containing Chitosan/Gelatin Coatings with Immunomodulatory Effect for Soft Tissue Sealing around Dental Implants
- A literature review on implant abutment and soft tissue response