J Periodontal Implant Sci.
2015 Jun;45(3):111-119. 10.5051/jpis.2015.45.3.111.
Effect of fibroblast growth factor on injured periodontal ligament and cementum after tooth replantation in dogs
- Affiliations
-
- 1Department of Periodontology, School of Dentistry, Chosun University, Gwangju, Korea.
- 2Department of Periodontology, Research Institute for Periodontal Regeneration, College of Dentistry, Yonsei University, Seoul, Korea. shchoi726@yuhs.ac
- 3Department of Oral Histology-Developmental Biology and Dental Research Institute, School of Dentistry, Seoul National University, Seoul, Korea.
- KMID: 1824940
- DOI: http://doi.org/10.5051/jpis.2015.45.3.111
Abstract
- PURPOSE
The purpose of this animal study was to perform a histological and histomorphometric analysis in order to elucidate the effect of fibroblast growth factor-2 (FGF-2) on injured periodontal ligament (PDL) and cementum after tooth replantation in dogs.
METHODS
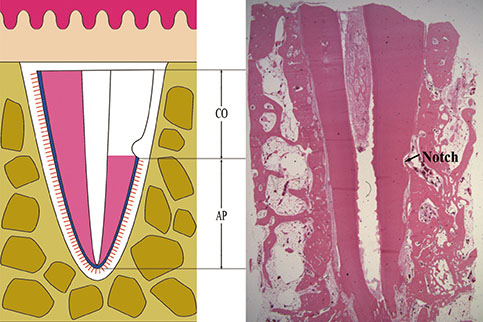
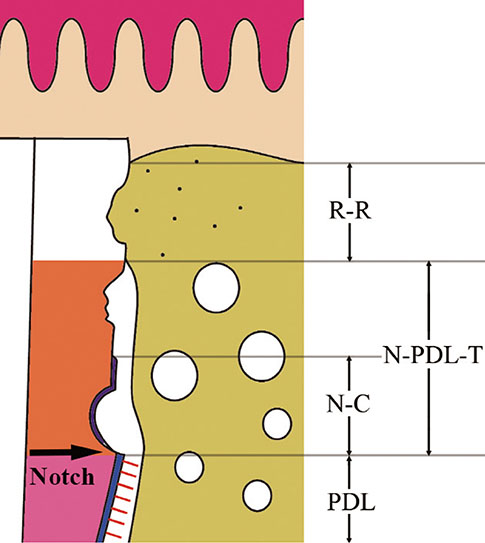
The roots of 36 mandibular premolars from six mongrel dogs were used in this study. The roots were randomly divided into three groups: (1) a positive control group (n=12), in which the PDL was retained; (2) a negative control group (n=12), in which the PDL and the cementum between the notches were removed; and (3) an experimental group (n=12), in which the PDL and the cementum between the notches were removed and the roots were soaked in an FGF-2 solution (30 microg/0.1 mL). After treating the root surfaces, the extracted roots were replanted into extraction sockets. The animals were sacrificed four and eight weeks after surgery for histologic and histomorphometric evaluation.
RESULTS
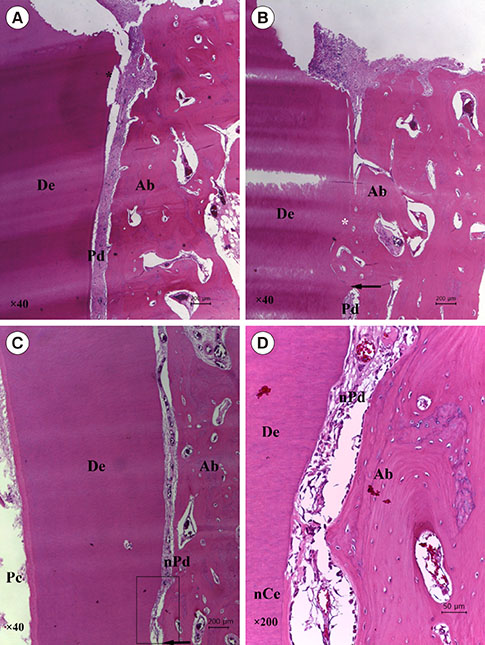
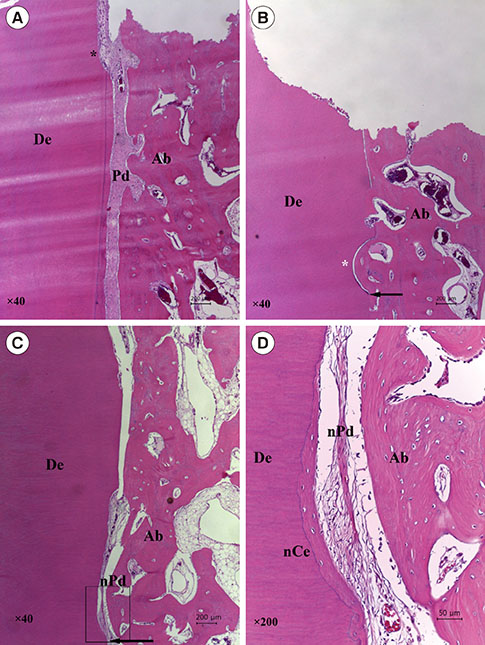
At four and eight weeks, normal PDLs covered the roots in the positive control group. In the negative control group, most replanted roots showed signs of replacement resorption. In the experimental group, new PDL-like tissue and cementum-like tissue were observed to partially occupy the region between the root surfaces and the newly formed bone. Histomorphometric analysis showed that the mean length of the newly formed cementum-like tissue on the roots treated with FGF-2 was significantly greater than that of the tissue on the roots in the negative control group (four weeks, P=0.008; eight weeks, P=0.042). However, no significant differences were observed between the roots treated with FGF-2 and the negative control roots with respect to newly formed PDL-like tissue.
CONCLUSIONS
The results of this study suggest that use of FGF-2 on injured root surfaces promotes cementogenesis after tooth replacement in dogs.
MeSH Terms
Figure
Reference
-
1. Kim E, Jung JY, Cha IH, Kum KY, Lee SJ. Evaluation of the prognosis and causes of failure in 182 cases of autogenous tooth transplantation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005; 100:112–119.
Article2. Temmerman L, De Pauw GA, Beele H, Dermaut LR. Tooth transplantation and cryopreservation: state of the art. Am J Orthod Dentofacial Orthop. 2006; 129:691–695.
Article3. Lee EU, Lim HC, Lee JS, Jung UW, Kim US, Lee SJ, et al. Delayed intentional replantation of periodontally hopeless teeth: a retrospective study. J Periodontal Implant Sci. 2014; 44:13–19.
Article4. Andreasen JO. Effect of extra-alveolar period and storage media upon periodontal and pulpal healing after replantation of mature permanent incisors in monkeys. Int J Oral Surg. 1981; 10:43–53.
Article5. Löe H, Waerhaug J. Experimental replantation of teeth in dogs and monkeys. Arch Oral Biol. 1961; 3:176–184.
Article6. Andreasen JO. Experimental dental traumatology: development of a model for external root resorption. Endod Dent Traumatol. 1987; 3:269–287.
Article7. Beertsen W, McCulloch CA, Sodek J. The periodontal ligament: a unique, multifunctional connective tissue. Periodontol 2000. 1997; 13:20–40.
Article8. Andreasen JO. Relationship between cell damage in the periodontal ligament after replantation and subsequent development of root resorption. A time-related study in monkeys. Acta Odontol Scand. 1981; 39:15–25.
Article9. Andreasen JO, Kristerson L. Evaluation of different types of autotransplanted connective tissues as potential periodontal ligament substitutes. An experimental replantation study in monkeys. Int J Oral Surg. 1981; 10:189–201.
Article10. Akbay A, Baran C, Günhan O, Ozmeriç N, Baloş K. Periodontal regenerative potential of autogenous periodontal ligament grafts in Class II furcation defects. J Periodontol. 2005; 76:595–604.
Article11. Modino SA, Sharpe PT. Tissue engineering of teeth using adult stem cells. Arch Oral Biol. 2005; 50:255–258.
Article12. Hynes K, Menicanin D, Gronthos S, Bartold PM. Clinical utility of stem cells for periodontal regeneration. Periodontol 2000. 2012; 59:203–227.
Article13. Melcher AH. On the repair potential of periodontal tissues. J Periodontol. 1976; 47:256–260.
Article14. Zhou Y, Hutmacher DW, Sae-Lim V, Zhou Z, Woodruff M, Lim TM. Osteogenic and adipogenic induction potential of human periodontal cells. J Periodontol. 2008; 79:525–534.
Article15. Okamoto T, Yatsuzuka N, Tanaka Y, Kan M, Yamanaka T, Sakamoto A, et al. Growth and differentiation of periodontal ligament-derived cells in serum-free defined culture. In Vitro Cell Dev Biol Anim. 1997; 33:302–309.
Article16. Nishimura F, Terranova VP. Comparative study of the chemotactic responses of periodontal ligament cells and gingival fibroblasts to polypeptide growth factors. J Dent Res. 1996; 75:986–992.
Article17. Murakami S, Takayama S, Ikezawa K, Shimabukuro Y, Kitamura M, Nozaki T, et al. Regeneration of periodontal tissues by basic fibroblast growth factor. J Periodontal Res. 1999; 34:425–430.
Article18. Takayama S, Murakami S, Shimabukuro Y, Kitamura M, Okada H. Periodontal regeneration by FGF-2 (bFGF) in primate models. J Dent Res. 2001; 80:2075–2079.
Article19. Murakami S, Takayama S, Kitamura M, Shimabukuro Y, Yanagi K, Ikezawa K, et al. Recombinant human basic fibroblast growth factor (bFGF) stimulates periodontal regeneration in class II furcation defects created in beagle dogs. J Periodontal Res. 2003; 38:97–103.
Article20. Humphrey JM, Kenny DJ, Barrett EJ. Clinical outcomes for permanent incisor luxations in a pediatric population. I. Intrusions. Dent Traumatol. 2003; 19:266–273.
Article21. Seshima F, Ota M, Kinumatsu T, Shibukawa Y, Yamada S. Effect of recombinant basic fibroblast growth factor on reimplanted teeth in beagle dogs. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010; 109:142–148.
Article22. Shiratani S, Ota M, Fujita T, Seshima F, Yamada S, Saito A. Effect of basic fibroblast growth factor on root resorption after delayed autotransplantation of tooth in dogs. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012; 114:e14–e21.
Article23. Sato Y, Kikuchi M, Ohata N, Tamura M, Kuboki Y. Enhanced cementum formation in experimentally induced cementum defects of the root surface with the application of recombinant basic fibroblast growth factor in collagen gel in vivo. J Periodontol. 2004; 75:243–248.
Article24. Schwartz O, Andreasen JO. Allo- and autotransplantation of mature teeth in monkeys: a sequential time-related histoquantitative study of periodontal and pulpal healing. Dent Traumatol. 2002; 18:246–261.
Article25. Söder PO, Otteskog P, Andreasen JO, Modéer T. Effect of drying on viability of periodontal membrane. Scand J Dent Res. 1977; 85:164–168.
Article26. Iqbal MK, Bamaas N. Effect of enamel matrix derivative (EMDOGAIN) upon periodontal healing after replantation of permanent incisors in beagle dogs. Dent Traumatol. 2001; 17:36–45.
Article27. Gopikrishna V, Thomas T, Kandaswamy D. A quantitative analysis of coconut water: a new storage media for avulsed teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008; 105:e61–e65.
Article28. Thomas T, Gopikrishna V, Kandaswamy D. Comparative evaluation of maintenance of cell viability of an experimental transport media "coconut water" with Hank's balanced salt solution and milk, for transportation of an avulsed tooth: An in vitro cell culture study. J Conserv Dent. 2008; 11:22–29.
Article29. Jung IH, Yun JH, Cho AR, Kim CS, Chung WG, Choi SH. Effect of (-)-epigallocatechin-3-gallate on maintaining the periodontal ligament cell viability of avulsed teeth: a preliminary study. J Periodontal Implant Sci. 2011; 41:10–16.
Article30. Goswami M, Chaitra T, Chaudhary S, Manuja N, Sinha A. Strategies for periodontal ligament cell viability: An overview. J Conserv Dent. 2011; 14:215–220.
Article31. Malhotra N. Current developments in interim transport (storage) media in dentistry: an update. Br Dent J. 2011; 211:29–33.
Article32. Moreira-Neto JJ, Gondim JO, Raddi MS, Pansani CA. Viability of human fibroblasts in coconut water as a storage medium. Int Endod J. 2009; 42:827–830.
Article33. Andreasen JO. Periodontal healing after replantation and autotransplantation of incisors in monkeys. Int J Oral Surg. 1981; 10:54–61.
Article34. Goerig AC, Nagy WW. Successful intentional reimplantation of mandibular molars. Quintessence Int. 1988; 19:585–588.35. Nethander G. Periodontal conditions of teeth autogenously transplanted by a two-stage technique. J Periodontal Res. 1994; 29:250–258.
Article36. Northway W. Autogenic dental transplants. Am J Orthod Dentofacial Orthop. 2002; 121:592–593.
Article37. Andersson L. Dentoalveolar ankylosis and associated root resorption in replanted teeth. Experimental and clinical studies in monkeys and man. Swed Dent J Suppl. 1988; 56:1–75.38. Mine K, Kanno Z, Muramoto T, Soma K. Occlusal forces promote periodontal healing of transplanted teeth and prevent dentoalveolar ankylosis: an experimental study in rats. Angle Orthod. 2005; 75:637–644.39. Yang Y, Bai Y, Li S, Li J, Gao W, Ru N. Effect of early orthodontic force on periodontal healing after autotransplantation of permanent incisors in beagle dogs. J Periodontol. 2012; 83:235–241.
Article40. Klagsbrun M, Smith S, Sullivan R, Shing Y, Davidson S, Smith JA, et al. Multiple forms of basic fibroblast growth factor: amino-terminal cleavages by tumor cell- and brain cell-derived acid proteinases. Proc Natl Acad Sci USA. 1987; 84:1839–1843.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of platelet-rich plasma on tooth replantation in dogs: a histologic and histomorphometric analysis
- Analysis of PDL Fibroblast Change During Mechanical Stimuli in the Rats
- Histologic changes of tooth and periodontal tissues applying to contraction & intrusion force for the maxillary four incisors of dogs
- Tissue changes of pulp and periodontium on rapid tooth movement with osteotomy in dogs
- Understanding of Cementum Formation by the Wnt/β-Catenin Signaling