J Periodontal Implant Sci.
2018 Aug;48(4):224-235. 10.5051/jpis.2018.48.4.224.
Effects of platelet-rich plasma on tooth replantation in dogs: a histologic and histomorphometric analysis
- Affiliations
-
- 1Department of Periodontology, Chosun University School of Dentistry, Gwangju, Korea. sjyu78@chosun.ac.kr
- 2Department of Periodontology, Research Institute for Periodontal Regeneration, Yonsei University School of Dentistry, Seoul, Korea.
- 3Department of Oral Histology/Developmental Biology, Dental Research Institute, Seoul National University School of Dentistry, Seoul, Korea.
- KMID: 2465388
- DOI: http://doi.org/10.5051/jpis.2018.48.4.224
Abstract
- PURPOSE
The purpose of this study was to evaluate the effects of platelet-rich plasma (PRP) on periodontal healing of replanted root surfaces in dogs histologically and histomorphometrically.
METHODS
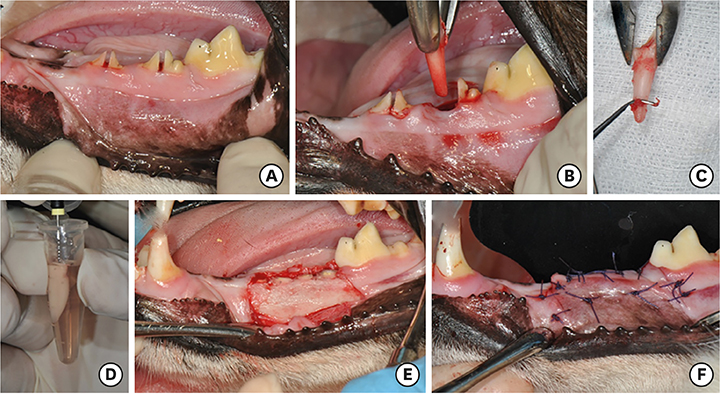
A total of 36 roots of mandibular incisors and premolars from 6 mongrel dogs were used. The roots were randomly divided into 3 groups: 1) a positive control group (n=12), in which the periodontal ligament (PDL) and cementum were retained and the roots were soaked in saline; 2) a negative control group (n=12), in which the PDL and cementum were removed and the roots were soaked in saline; and 3) an experimental group (n=12), in which the PDL and cementum were removed and the roots were soaked in PRP. After soaking the root surfaces, the extracted roots were replanted into the extraction sockets. The roots were covered using a coronally repositioned flap
RESULTS
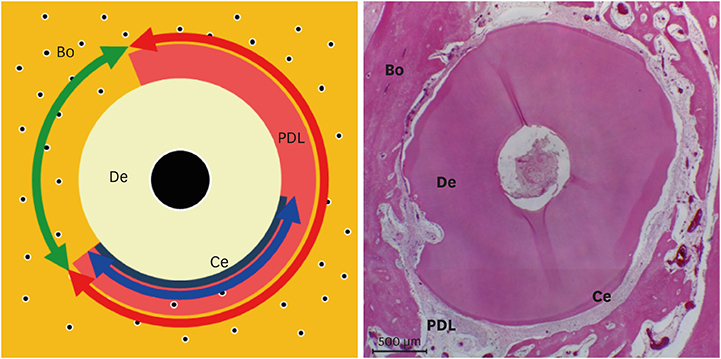
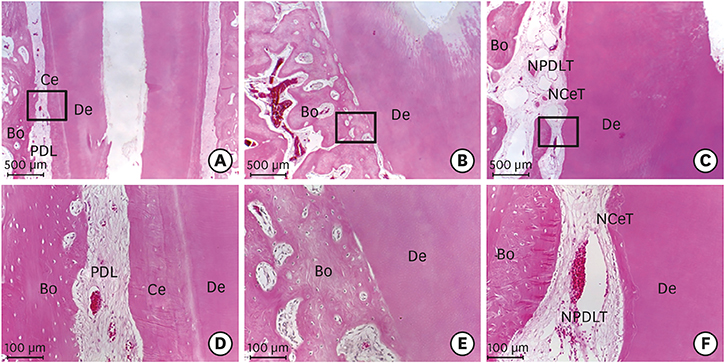
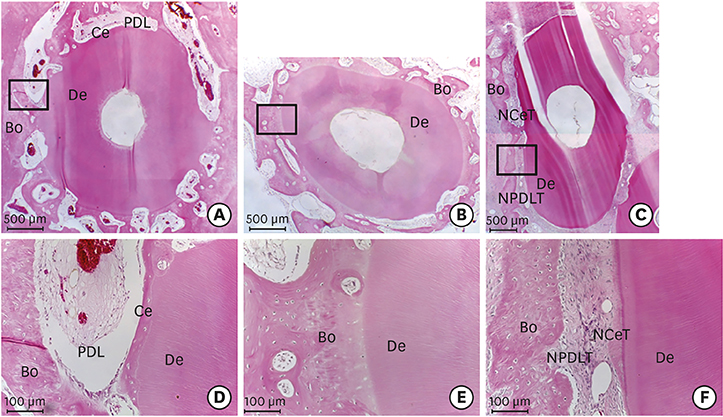
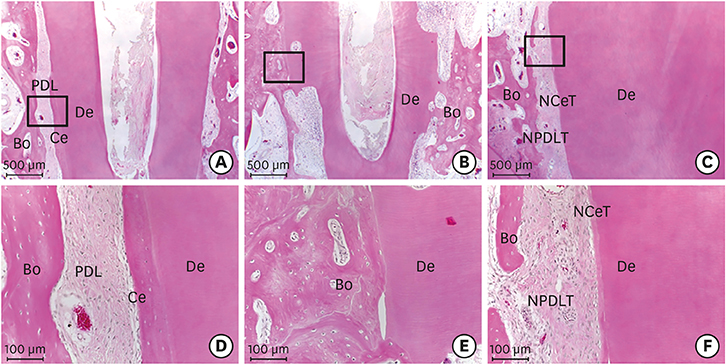
Histologically, irregular-thickness PDL-like and cementum-like tissues were observed in the 4-week experimental group and the positive control group. PDL-like tissue and cementum-like tissue with a more uniform thickness were observed at 8 weeks. In the negative control group, PDL-like tissue and cementum-like tissue were rarely found, and root resorption and ankylosis were observed. In the cross-sectional histomorphometric analysis, the experimental group demonstrated a higher rate of formation of cementum-like tissue and a lower tooth ankylosis rate than the positive and negative control groups at 4 and 8 weeks. Although there was a significant difference in the tooth ankylosis rate and the formation of cementum-like tissue across the 3 groups (P < 0.05), no statistical significance was observed between any pair of groups (P > 0.017).
CONCLUSIONS
Applying PRP to root surfaces during tooth replantation in dogs can reduce tooth ankylosis and increase PDL-like and cementum-like tissue formation.
Keyword
MeSH Terms
Figure
Reference
-
1. Grossman LI. Intentional replantation of teeth. J Am Dent Assoc. 1966; 72:1111–1118.
Article2. Madison S. Intentional replantation. Oral Surg Oral Med Oral Pathol. 1986; 62:707–709.
Article3. Weine FS. The case against intentional replantation. J Am Dent Assoc. 1980; 100:664–668.
Article4. Lu DP. Intentional replantation of periodontally involved and endodontically mistreated tooth. Oral Surg Oral Med Oral Pathol. 1986; 61:508–513.
Article5. Demiralp B, Nohutçu RM, Tepe DI, Eratalay K. Intentional replantation for periodontally involved hopeless teeth. Dent Traumatol. 2003; 19:45–51.
Article6. Fréchette JP, Martineau I, Gagnon G. Platelet-rich plasmas: growth factor content and roles in wound healing. J Dent Res. 2005; 84:434–439.
Article7. Cho MI, Lin WL, Genco RJ. Platelet-derived growth factor-modulated guided tissue regenerative therapy. J Periodontol. 1995; 66:522–530.
Article8. Park JB, Matsuura M, Han KY, Norderyd O, Lin WL, Genco RJ, et al. Periodontal regeneration in class III furcation defects of beagle dogs using guided tissue regenerative therapy with platelet-derived growth factor. J Periodontol. 1995; 66:462–477.
Article9. Jin Q, Anusaksathien O, Webb SA, Printz MA, Giannobile WV. Engineering of tooth-supporting structures by delivery of PDGF gene therapy vectors. Mol Ther. 2004; 9:519–526.
Article10. Saygin NE, Tokiyasu Y, Giannobile WV, Somerman MJ. Growth factors regulate expression of mineral associated genes in cementoblasts. J Periodontol. 2000; 71:1591–1600.
Article11. Takayama S, Murakami S, Miki Y, Ikezawa K, Tasaka S, Terashima A, et al. Effects of basic fibroblast growth factor on human periodontal ligament cells. J Periodontal Res. 1997; 32:667–675.
Article12. Terranova VP, Odziemiec C, Tweden KS, Spadone DP. Repopulation of dentin surfaces by periodontal ligament cells and endothelial cells. Effect of basic fibroblast growth factor. J Periodontol. 1989; 60:293–301.
Article13. Fujii S, Maeda H, Tomokiyo A, Monnouchi S, Hori K, Wada N, et al. Effects of TGF-β1 on the proliferation and differentiation of human periodontal ligament cells and a human periodontal ligament stem/progenitor cell line. Cell Tissue Res. 2010; 342:233–242.
Article14. Moore YR, Dickinson DP, Wikesjö UM. Growth/differentiation factor-5: a candidate therapeutic agent for periodontal regeneration? A review of pre-clinical data. J Clin Periodontol. 2010; 37:288–298.
Article15. Cáceres M, Hidalgo R, Sanz A, Martínez J, Riera P, Smith PC. Effect of platelet-rich plasma on cell adhesion, cell migration, and myofibroblastic differentiation in human gingival fibroblasts. J Periodontol. 2008; 79:714–720.
Article16. Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998; 85:638–646.17. Tözüm TF, Demiralp B. Platelet-rich plasma: a promising innovation in dentistry. J Can Dent Assoc. 2003; 69:664.18. Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part II: platelet-related biologic features. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006; 101:e45–e50.
Article19. Shanaman R, Filstein MR, Danesh-Meyer MJ. Localized ridge augmentation using GBR and platelet-rich plasma: case reports. Int J Periodontics Restorative Dent. 2001; 21:345–355.20. Wiltfang J, Schlegel KA, Schultze-Mosgau S, Nkenke E, Zimmermann R, Kessler P. Sinus floor augmentation with β-tricalciumphosphate (β-TCP): does platelet-rich plasma promote its osseous integration and degradation? Clin Oral Implants Res. 2003; 14:213–218.
Article21. Camargo PM, Lekovic V, Weinlaender M, Vasilic N, Madzarevic M, Kenney EB. Platelet-rich plasma and bovine porous bone mineral combined with guided tissue regeneration in the treatment of intrabony defects in humans. J Periodontal Res. 2002; 37:300–306.
Article22. Srinivas BV, Rupa N, Halini Kumari KV, Prasad SS, Varalakshmi U, Sudhakar K. Root coverage using subepithelial connective tissue graft with platelet-rich plasma in the treatment of gingival recession: a clinical study. J Pharm Bioallied Sci. 2015; 7:S530–S538.
Article23. Petrungaro PS. Treatment of the infected implant site using platelet-rich plasma. Compend Contin Educ Dent. 2002; 23:363–366. 36824. Polimeni G, Xiropaidis AV, Wikesjö UM. Biology and principles of periodontal wound healing/regeneration. Periodontol 2000. 2006; 41:30–47.
Article25. Chen FM, Chen R, Wang XJ, Sun HH, Wu ZF. In vitro cellular responses to scaffolds containing two microencapulated growth factors. Biomaterials. 2009; 30:5215–5224.
Article26. Park SY, Kim KH, Shin SY, Koo KT, Lee YM, Seol YJ. Dual delivery of rhPDGF-BB and bone marrow mesenchymal stromal cells expressing the BMP2 gene enhance bone formation in a critical-sized defect model. Tissue Eng Part A. 2013; 19:2495–2505.
Article27. Chan CP, Lin CP, Chang MC, Hsieh CC, Hsu CC, Lin CL, et al. Effects of thrombin on the growth, protein synthesis, attachment, clustering and alkaline phosphatase activity of cultured human periodontal ligament fibroblasts. Proc Natl Sci Counc Repub China B. 1998; 22:137–143.28. Reichert da Silva Assunção L, Colenci R, Ferreira do-Amaral CC, Sonoda CK, Mogami Bomfim SR, Okamoto R, et al. Periodontal tissue engineering after tooth replantation. J Periodontol. 2011; 82:758–766.
Article29. Zhao YH, Zhang M, Liu NX, Lv X, Zhang J, Chen FM, et al. The combined use of cell sheet fragments of periodontal ligament stem cells and platelet-rich fibrin granules for avulsed tooth reimplantation. Biomaterials. 2013; 34:5506–5520.
Article30. Andersson L, Jonsson BG, Hammarström L, Blomlöf L, Andreasen JO, Lindskog S. Evaluation of statistics and desirable experimental design of a histomorphometrical method for studies of root resorption. Endod Dent Traumatol. 1987; 3:288–295.
Article31. Andreasen JO. Effect of extra-alveolar period and storage media upon periodontal and pulpal healing after replantation of mature permanent incisors in monkeys. Int J Oral Surg. 1981; 10:43–53.
Article32. Andreasen JO, Borum MK, Jacobsen HL, Andreasen FM. Replantation of 400 avulsed permanent incisors. 4. Factors related to periodontal ligament healing. Endod Dent Traumatol. 1995; 11:76–89.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Platelet Rich Plasma Combined with Bovine Bone on the Treatment of Grade II Furcation Defects in Beagle Dogs
- The effect of platelet rich plasma in bone formation on implant installation in the tibia of beagle dogs
- Salvage of Unilateral Complete Ear Amputation with Continuous Local Hyperbaric Oxygen, Platelet-Rich Plasma and Polydeoxyribonucleotide without Micro-Revascularization
- Effect of Platelet-rich Plasma on Burn Wounds according to Time of Application: An Experimental Study on Rats
- Effect of fibroblast growth factor on injured periodontal ligament and cementum after tooth replantation in dogs