J Korean Surg Soc.
2011 Apr;80(4):289-296. 10.4174/jkss.2011.80.4.289.
Association with inflammatory cells and apolipoproteins to the progression of atherosclerosis
- Affiliations
-
- 1Division of Vascular Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. dikim@skku.edu
- KMID: 1820021
- DOI: http://doi.org/10.4174/jkss.2011.80.4.289
Abstract
- PURPOSE
Inflammatory cells are known to be associated with the progression of atherosclerosis and plaque rupture. However, the relation to inflammatory cells and apolipoproteins on the progression of atherosclerosis is unknown. This study was aimed at examining the different expressions of inflammatory cells and evaluate the effect of apolipoprotein (APO) C1 and APO E during the progression of atherosclerosis.
METHODS
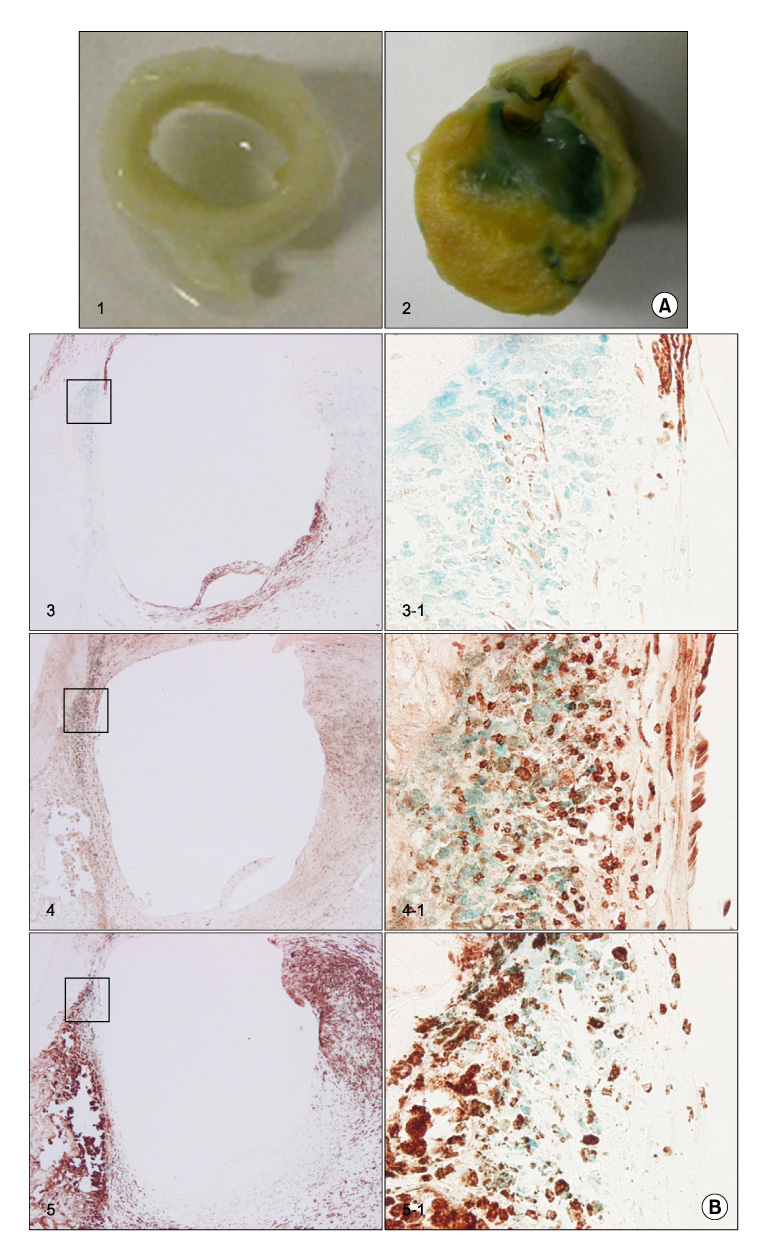
Ten atherosclerotic tissues were compared with five non-atherosclerotic tissues. The presence of vascular smooth muscle cells (VSMCs), macrophages, T-cells, APO C1, and APO E were identified by Western blotting and immunohistochemical analysis with antibodies. The senescence was analyzed by senescence-associated beta-galactosidase.
RESULTS
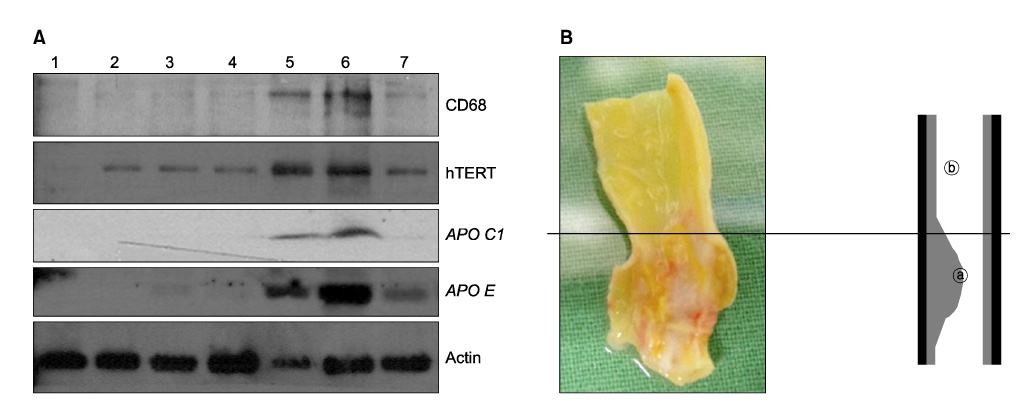
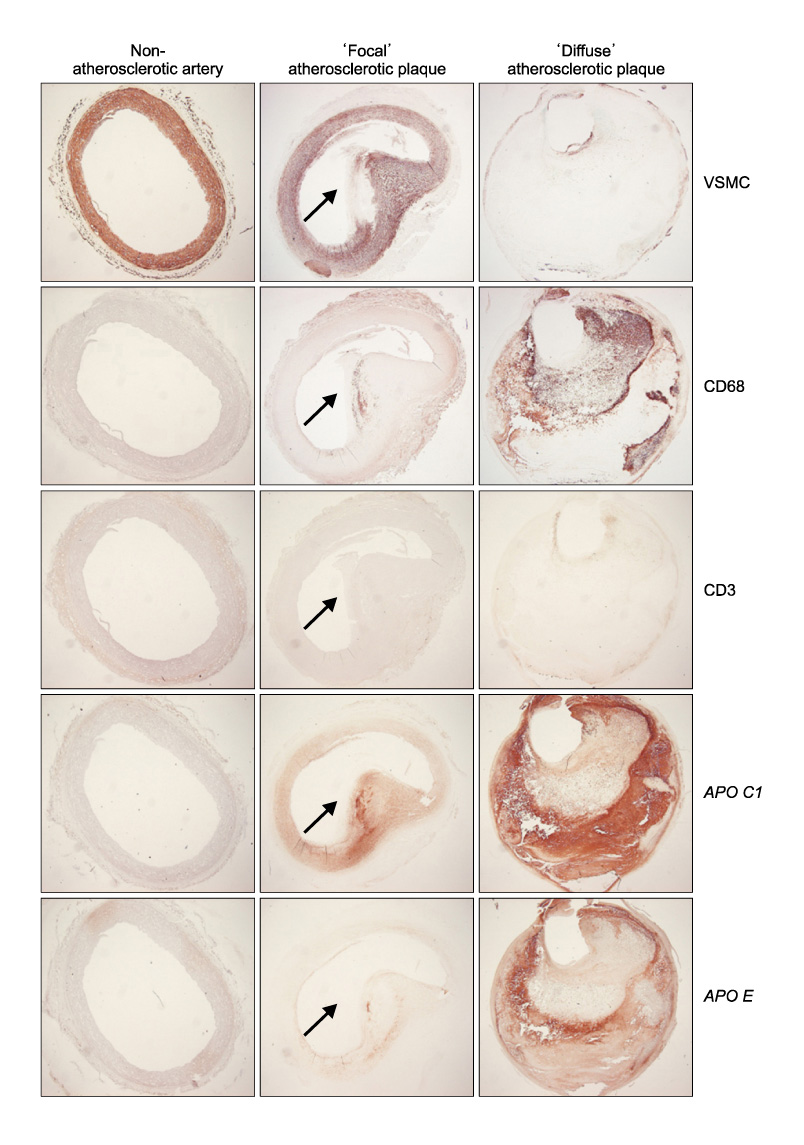
The protein expression and senescence of macrophages, APO C1 and APO E were significantly higher in the main atherosclerotic lesion than the non-atherosclerotic lesion. A high concentration of inflammatory cells and the paucity of VSMCs were present in the shoulder area. In addition, macrophage and T-cells are expressed in the early stage of atherosclerotic development and more expanded in advanced atherosclerotic plaques. APO C1 was expressed mainly within the necrotic core, and APO E existed mostly around the necrotic core and the fibrous cap in advanced atherosclerotic plaques.
CONCLUSION
Our study indicated that the expression and the senescence of macrophage and T-cells may be closelyrelated to induction and deposition of APO C1 and APO E. This contributes to the development and progression of atherosclerotic plaque by expanding the necrotic core.
MeSH Terms
Figure
Reference
-
1. Gordon D, Reidy MA, Benditt EP, Schwartz SM. Cell proliferation in human coronary arteries. Proc Natl Acad Sci U S A. 1990. 87:4600–4604.2. O'Brien ER, Alpers CE, Stewart DK, Ferguson M, Tran N, Gordon D, et al. Proliferation in primary and restenotic coronary atherectomy tissue. Implications for antiproliferative therapy. Circ Res. 1993. 73:223–231.3. Katsuda S, Coltrera MD, Ross R, Gown AM. Human atherosclerosis. IV. Immunocytochemical analysis of cell activation and proliferation in lesions of young adults. Am J Pathol. 1993. 142:1787–1793.4. Kockx MM, Herman AG. Apoptosis in atherosclerosis: beneficial or detrimental? Cardiovasc Res. 2000. 45:736–746.5. Leskinen MJ, Kovanen PT, Lindstedt KA. Regulation of smooth muscle cell growth, function and death in vitro by activated mast cells--a potential mechanism for the weakening and rupture of atherosclerotic plaques. Biochem Pharmacol. 2003. 66:1493–1498.6. Lutgens E, de Muinck ED, Kitslaar PJ, Tordoir JH, Wellens HJ, Daemen MJ. Biphasic pattern of cell turnover characterizes the progression from fatty streaks to ruptured human atherosclerotic plaques. Cardiovasc Res. 1999. 41:473–479.7. Ross R, Wight TN, Strandness E, Thiele B. Human atherosclerosis. I. Cell constitution and characteristics of advanced lesions of the superficial femoral artery. Am J Pathol. 1984. 114:79–93.8. Bennett MR, Evan GI, Schwartz SM. Apoptosis of human vascular smooth muscle cells derived from normal vessels and coronary atherosclerotic plaques. J Clin Invest. 1995. 95:2266–2274.9. Geng YJ, Libby P. Evidence for apoptosis in advanced human atheroma. Colocalization with interleukin-1 beta-converting enzyme. Am J Pathol. 1995. 147:251–266.10. Clarke M, Bennett M. Defining the role of vascular smooth muscle cell apoptosis in atherosclerosis. Cell Cycle. 2006. 5:2329–2331.11. Bennett MR, Boyle JJ. Apoptosis of vascular smooth muscle cells in atherosclerosis. Atherosclerosis. 1998. 138:3–9.12. Lauer SJ, Walker D, Elshourbagy NA, Reardon CA, Levy-Wilson B, Taylor JM. Two copies of the human apolipoprotein C-I gene are linked closely to the apolipoprotein E gene. J Biol Chem. 1988. 263:7277–7286.13. Hashimoto S, Suzuki T, Dong HY, Yamazaki N, Matsushima K. Serial analysis of gene expression in human monocytes and macrophages. Blood. 1999. 94:837–844.14. Eo HS, Kim DI. Apolipoprotein C1 and apolipoprotein E are differentially expressed in atheroma of the carotid and femoral artery. J Surg Res. 2008. 144:132–137.15. Libby P. Changing concepts of atherogenesis. J Intern Med. 2000. 247:349–358.16. Rees D, Sloane T, Jessup W, Dean RT, Kritharides L. Apolipoprotein A-I stimulates secretion of apolipoprotein E by foam cell macrophages. J Biol Chem. 1999. 274:27925–27933.17. Piedrahita JA, Zhang SH, Hagaman JR, Oliver PM, Maeda N. Generation of mice carrying a mutant apolipoprotein E gene inactivated by gene targeting in embryonic stem cells. Proc Natl Acad Sci U S A. 1992. 89:4471–4475.18. Nakashima Y, Plump AS, Raines EW, Breslow JL, Ross R. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler Thromb. 1994. 14:133–140.19. Poch E, Carbonell P, Franco S, Díez-Juan A, Blasco MA, Andrés V. Short telomeres protect from diet-induced atherosclerosis in apolipoprotein E-null mice. FASEB J. 2004. 18:418–420.20. Shiomi M, Ito T, Tsukada T, Yata T, Ueda M. Cell compositions of coronary and aortic atherosclerotic lesions in WHHL rabbits differ. An immunohistochemical study. Arterioscler Thromb. 1994. 14:931–937.21. Davies MJ, Richardson PD, Woolf N, Katz DR, Mann J. Risk of thrombosis in human atherosclerotic plaques: role of extracellular lipid, macrophage, and smooth muscle cell content. Br Heart J. 1993. 69:377–381.22. Kockx MM, De Meyer GR, Bortier H, de Meyere N, Muhring J, Bakker A, et al. Luminal foam cell accumulation is associated with smooth muscle cell death in the intimal thickening of human saphenous vein grafts. Circulation. 1996. 94:1255–1262.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Metabolic Reprogramming of Macrophages in Atherosclerosis: Is It All about Cholesterol?
- Deciphering Macrophage Phenotypes upon Lipid Uptake and Atherosclerosis
- Current Understanding of Dendritic Cells in Atherosclerosis
- Pathophysiology of Atherosclerosis
- C-Reactive Protein Signaling Pathways in Tumor Progression