Cancer Res Treat.
2005 Aug;37(4):223-227.
A Feasibility Study of Adenosine Triphosphate-based Chemotherapy Response Assay (ATP-CRA) as a Chemosensitivity Test for Lung Cancer
- Affiliations
-
- 1Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea. kjhang@yumc.yonsei.ac.kr
- 2Department of Pathology, Yonsei University College of Medicine, Seoul, Korea.
- 3Department of Cardiovascular and Thoracic Surgery, Yonsei University College of Medicine, Seoul, Korea.
- 4Yonsei Cancer Center, Yonsei University College of Medicine, Seoul, Korea.
- 5ISU ABXIS CO., LTD, Seoul, Korea.
Abstract
- PURPOSE
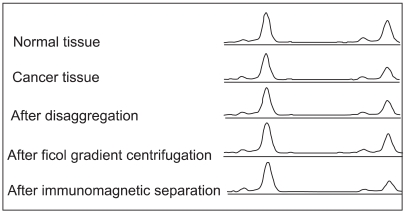
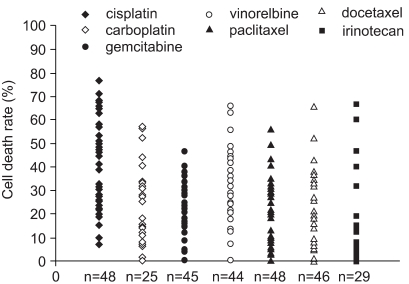
A chemosensitivity test can reflect the differences in responses of individual cancer patients to chemotherapeutic agents. The adenosine triphosphate-based chemotherapy response assay (ATP-CRA)is an accurate method, which does not require a large amount of tissue specimen. So far, no studies have evaluated the utility of the ATP-CRA in Korea. Therefore, we investigated the clinical usefulness of the ATP-CRA in 53 patients with lung cancer. MATERIALS AND METHODS: Tumor tissues were obtained from bronchoscopic biopsies or surgical resections. The validity of ATP-CRA was assessed focusing on the success rate, experimental error level (intraassay mean coefficient of variation [CV]) and reproducibility. RESULTS: The overall success rate of ATP-CRA was 90.6% (48/53). Normal cells were effectively eliminated from the tumor tissues with the use of ficoll gradient centrifugation and immunomagnetic separation, which was confirmed using loss of heterozygosity analysis of the 3p deletion. The mean CV of ATP assays was 10.5+/-4.6%. The reproducibility of ATP assays was 94+/-3.8%. The results of the ATP assays were reported to physicians within 7 days of specimen collection. More than 6 anticancer drugs were tested on the tumor specimens obtained from bronchoscopic biopsies. CONCLUSION: The ATP-CRA is a stable, accurate and potentially practical chemosensitivity test in patients with lung cancer.
Keyword
MeSH Terms
Figure
Reference
-
1. National Statistics Office. Annual Report of Death Statistics Data in 2003. Korea:2. The International Adjuvant Lung Cancer Trial Collaborative Group. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004; 350:351–360. PMID: 14736927.3. Kato H, Ichinose Y, Ohta M, Hata E, Tsubota N, Tada H, et al. A randomized trial of adjuvant chemotherapy with uracil-tegafur for adenocarcinoma of the lung. N Engl J Med. 2004; 350:1713–1721. PMID: 15102997.
Article4. Salmon SE, Hamburger AW, Soehnlen B, Durie BG, Alberts DS, Moon TE. Quantitation of differential sensitivity of human-tumor stem cells to anticancer drugs. N Engl J Med. 1978; 298:1321–1327. PMID: 77475.
Article5. Maehara Y, Anai H, Tamada R, Sugimachi K. The ATP assay is more sensitive than the succinate dehydrogenase inhibition test for predicting cell viability. Eur J Cancer Clin Oncol. 1987; 23:273–276. PMID: 3109921.
Article6. Petty RD, Sutherland LA, Hunter EM, Cree IA. Comparison of MTT and ATP-based assays for the measurement of viable cell number. J Biolumin Chemilumin. 1995; 10:29–34. PMID: 7762413.
Article7. Cree IA, Kurbacher CM. Individualizing chemotherapy for solid tumors-is there any alternative? Anticancer Drugs. 1997; 8:541–548. PMID: 9300568.8. Andreotti PE, Cree IA, Kurbacher CM, Hartmann DM, Linder D, Harel G, et al. Chemosensitivity testing of human tumors using a microplate adenosine triphosphate luminescence assay: clinical correlation for cisplatin resistance of ovarian carcinoma. Cancer Res. 1995; 55:5276–5282. PMID: 7585588.9. Myatt N, Cree IA, Kurbacher CM, Foss AJ, Hungerford JL, Plowman PN. The ex vivo chemosensitivity profile of choroidal melanoma. Anticancer Drugs. 1997; 8:756–762. PMID: 9396619.
Article10. Cree IA, Neale MH, Myatt NE, de Takats PG, Hall P, Grant J, et al. Heterogeneity of chemosensitivity of metastatic cutaneous melanoma. Anticancer Drugs. 1999; 10:437–444. PMID: 10477162.
Article11. Breidenbach M, Rein D, Schmidt T, Heindel W, Kolhagen H, Mallmann P, et al. Intra-arterial mitoxantrone and paclitaxel in a patient with Stewart-Treves syndrome: selection of chemotherapy by an ex vivo ATP-based chemosensitivity assay. Anticancer Drugs. 2000; 11:269–273. PMID: 10898542.
Article12. Breidenbach M, Rein DT, Mallmann P, Kurbacher CM. Individualized long-term chemotherapy for recurrent ovarian cancer after failing high-dose treatment. Anticancer Drugs. 2002; 13:173–176. PMID: 11901311.
Article13. O'Meara AT, Sevin BU. Predictive value of the ATP chemosensitivity assay in epithelial ovarian cancer. Gynecol Oncol. 2001; 83:334–342. PMID: 11606094.14. Cree IA, Kurbacher CM, Untch M, Sutherland LA, Hunter EM, Subedi AM, et al. Correlation of the clinical response to chemotherapy in breast cancer with ex vivo chemosensitivity. Anticancer Drugs. 1996; 7:630–635. PMID: 8913430.
Article15. Kawamura H, Ikeda K, Takiyama I, Terashima M. The usefulness of the ATP assay with serum-free culture for chemosensitivity testing of gastrointestinal cancer. Eur J Cancer. 1997; 33:960–966. PMID: 9291821.
Article16. Sharma S, Neale MH, Di Nicolantonio F, Knight LA, Whitehouse PA, Mercer SJ, et al. Outcome of ATP-based tumor chemosensitivity assay directed chemotherapy in heavily pre-treated recurrent ovarian carcinoma. BMC Cancer. 2003; 3:19. PMID: 12841853.
Article17. Konecny G, Crohns C, Pegram M, Felber M, Lude S, Kurbacher C, et al. Correlation of drug response with the ATP tumorchemosensitivity assay in primary FIGO stage III ovarian cancer. Gynecol Oncol. 2000; 77:258–263. PMID: 10785475.
Article18. Kang HJ, Ko CD, Yoon HS, Kim MB, Ahn SH. The Reliability of histoculture drug response assay (HDRA) in chemosensitivity test for breast cancer. Cancer Res Treat. 2001; 33:392–397.19. DeVita VT, Hellman S, Rosenberg SA, editors. Principles and practice of oncology. 2001. 6th ed. Philadelphia: Lippincott Williams & Wilkins;p. 303.20. Cortazar P, Johnson BE. Review of the efficacy of individualized chemotherapy selected by in vitro drug sensitivity testing for patients with cancer. J Clin Oncol. 1999; 17:1625–1631. PMID: 10334552.
Article21. Ng TY, Ngan HY, Cheng DK, Wong LC. Clinical applicability of the ATP cell viability assay as a predictor of chemoresponse in platinum-resistant epithelial ovarian cancer using nonsurgical tumor cell samples. Gynecol Oncol. 2000; 76:405–408. PMID: 10684718.
Article22. Reinhold U, Tilgen W. Chemosensitivity testing in oncology. 2003. Heidelberg: Springer-Verlag;p. 126–145.23. Yamaue H, Tanimura H, Tsunoda T, Tani M, Iwahashi M, Noguchi K, et al. Chemosensitivity testing with highly purified fresh human tumour cells with the MTT colorimetric assay. Eur J Cancer. 1991; 7:1258–1263. PMID: 1835595.
Article24. Maehara Y, Kusumoto H, Kusumoto T, Anai H, Sugimachi K. Tumor tissue is more sensitive to mitomycin C, carboquone, and aclacinomycin A than is adjacent normal tissue in vitro. J Surg Oncol. 1989; 40:4–7. PMID: 2909804.
Article25. Kim BS, Ahn YM, Kim J, Yoon EJ, Choi SH. Clinical utility of adenosine triphosphate-based chemosensitivity response assay (ATP-CRA) in non-small cell lung cancer: preliminary study. Proc Am Soc Clin Oncol. 2004; 23:677.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- In-vitro Chemosensitivity Test for Colorectal Cancer using an Adenosine-triphosphate-based Chemotherapy Response Assay (ATP-CRA)
- Correlation between the molecular subtype of breast cancer and the in vitro adenosine triphosphate-based chemosensitivity assay
- Heterogeneity of Adenosine Triphosphate-Based Chemotherapy Response Assay in Colorectal Cancer - Secondary Publication
- In Vitro Adenosine Triphosphate Based Chemotherapy Response Assay in Gastric Cancer
- Heterogeneous Chemosensitivity of Breast Cancer Determined by Adeonsine Triphosphate Based Chemotherapy Response Assay