Cancer Res Treat.
2005 Aug;37(4):201-207.
Efficacy and Safety Study of Docetaxel as Salvage Chemotherapy in Metastatic Gastric Cancer Failing Fluoropyrimidine and Platinum Combination Chemotherapy
- Affiliations
-
- 1Division of Oncology, Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. ykkang@amc.seoul.kr
- 2Department of Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
Abstract
- PURPOSE
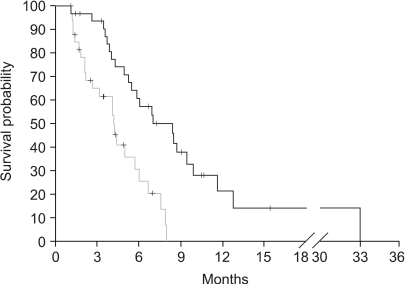
Fluoropyrimidine (F) and platinum (P) combination chemotherapy has been widely used for the first line treatment of advanced gastric cancer (AGC). Docetaxel (D) has shown promising activity in this disease. The present study retrospectively investigated the efficacy of D monotherapy as salvage chemotherapy for AGC that is failing F and P combination chemotherapy. MATERIALS AND METHODS: A total of 34 patients, fitting the eligibility criteria, were included in this study. D was administered at a dose of 75 mg/m2 IV every 3 weeks, with dexamethasone prophylaxis. Twenty-nine patients had measurable lesions. The median treatment-free interval was 38.5 days, and 91.2% of patients had progressed within 4 months of withdrawal of the first line chemotherapy. RESULTS: A total of 133 cycles of D were administered, with a median of 3.5 (1~8) cycles. From an intention-to-treat analysis, 6 patients achieved partial responses (PR), with a response rate of 20.7% (95% CI, 6.0~35.4). The duration of objective PRs in these six were 2.3+, 2.5+, 2.9, 3.0+, 6.2 and 6.8 months, respectively. Six patients showed a stable disease, but 15 showed progression. The median time to progression was 4.2 months (95% CI, 2.8~5.5), with a median overall survival since the start of D monotherapy of 8.4 months (95% CI, 5.5~11.3). Grade 3/4 neutropenia and febrile neutropenia occurred in 12.9% of patients and 3.1% of cycles. The incidence of grade 3 or worse non-hematological toxicities were as follows; peripheral sensory neuropathy 9.7%, asthenia 3.2% and allergic reaction 2.7%. CONCLUSION: Docetaxel, 75 mg/m2, is active in AGC as second-line chemotherapy after failure of prior exposure to the F and P combination chemotherapy, with a favorable toxicity profile.
Keyword
MeSH Terms
Figure
Reference
-
1. Parkin DM, Bray F, Ferlay J, Pisani P. Global Cancer Statistics, 2002. CA Cancer J Clin. 2005; 55:74–108. PMID: 15761078.
Article2. Shin HR, Jung KW, Won YJ, Park JG. 2002 annual report of the Korea Central Cancer Registry: based on registered data from 139 hospitals. Cancer Res Treat. 2004; 36:103–114.
Article3. Murad AM, Santiago FF, Petroianu A, Rocha PR, Rodrigues MA, Rausch M. Modified therapy with 5-fluorouracil, doxorubicin, and methotrexate in advanced gastric cancer. Cancer. 1993; 72:37–41. PMID: 8508427.
Article4. Glimelius B, Ekstrom K, Hoffman K, Graf W, Sjoden PO, Haglund U, et al. Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann Oncol. 1997; 8:163–168. PMID: 9093725.
Article5. Pyrhonen S, Kuitunen T, Nyandoto P, Kouri M. Randomised comparison of fluorouracil, epidoxorubicin and methotrexate (FEMTX) plus supportive care with supportive care alone in patients with non-resectable gastric cancer. Br J Cancer. 1995; 71:587–591. PMID: 7533517.
Article6. Waters JS, Norman A, Cunningham D, Scarffe JH, Webb A, Harper P, et al. Long-term survival after epirubicin, cisplatin and fluorouracil for gastric cancer: results of a randomized trial. Br J Cancer. 1999; 80:269–272. PMID: 10390007.
Article7. Webb A, Cunningham D, Scarffe JH, Harper P, Norman A, Joffe JK, et al. Randomized trial comparing epirubicin, cisplatin, and fluorouracil versus fluorouracil, doxorubicin, and methotrexate in advanced esophagogastric cancer. J Clin Oncol. 1997; 15:261–267. PMID: 8996151.
Article8. Kim NK, Park YS, Heo DS, Suh C, Kim SY, Park KC, et al. A phase III randomized study of 5-fluorouracil and cisplatin versus 5-fluorouracil, doxorubicin, and mitomycin C versus 5-fluorouracil alone in the treatment of advanced gastric cancer. Cancer. 1993; 71:3813–3818. PMID: 8508349.
Article9. Vanhoefer U, Rougier P, Wilke H, Ducreux MP, Lacave AJ, Van Cutsem E, et al. Final results of a randomized phase III trial of sequential high-dose methotrexate, fluorouracil, and doxorubicin versus etoposide, leucovorin, and fluorouracil versus infusional fluorouracil and cisplatin in advanced gastric cancer: a Trial of the European Organization for Research and Treatment of Cancer Gastrointestinal Tract Cancer Cooperative Group. J Clin Oncol. 2000; 18:2648–2657. PMID: 10894863.
Article10. Kim TW, Kang YK, Ahn JH, Chang HM, Yook JH, Oh ST, et al. Phase II study of capecitabine plus cisplatin as first-line chemotherapy in advanced gastric cancer. Ann Oncol. 2002; 13:1893–1898. PMID: 12453857.
Article11. Koizumi W, Tanabe S, Saigenji K, Ohtsu A, Boku N, Nagashima F, et al. Phase I/II study of S-1 combined with cisplatin in patients with advanced gastric cancer. Br J Cancer. 2003; 89:2207–2212. PMID: 14676796.
Article12. Sulkes A, Smyth J, Sessa C, Dirix LY, Vermorken JB, Kaye S, et al. EORTC Early Clinical Trials Group. Docetaxel (Taxotere) in advanced gastric cancer: results of a phase II clinical trial. Br J Cancer. 1994; 70:380–383. PMID: 7914428.13. Einzig AI, Neuberg D, Remick SC, Karp DD, O'Dwyer PJ, Stewart JA, et al. Phase II trial of docetaxel (Taxotere) in patients with adenocarcinoma of the upper gastrointestinal tract previously untreated with cytotoxic chemotherapy: the Eastern Cooperative Oncology Group (ECOG) results of protocol E1293. Med Oncol. 1996; 13:87–93. PMID: 9013471.
Article14. Giuliani F, Gebbia V, De Vita F, Maiello E, Di Bisceglie M, Catalano G, et al. Docetaxel as salvage therapy in advanced gastric cancer: a phase II study of the Gruppo Oncologico Italia Meridionale (G.O.I.M.). Anticancer Res. 2003; 23(5b):4219–4222. PMID: 14666628.15. Vanhoefer U, Wilke H, Harstrick A, Achterrath W, Preusser P, Stahl M, et al. Phase II study of docetaxel as second line chemotherapy in metastatic gastric cancer. Proc Am Soc Clin Oncol. 1999; 18:303. (abstr).16. Bang YJ, Kang WK, Kang YK, Kim HC, Jacques C, Zuber E, et al. Docetaxel 75 mg/m2 is active and well tolerated in patients with metastatic or recurrent gastric cancer: a phase II trial. Jpn J Clin Oncol. 2002; 32:248–254. PMID: 12324575.17. Taguchi T, Sakata Y, Kanamaru R, Kurihara M, Suminaga M, Ota J, et al. Late phase II clinical study of RP56976 (docetaxel) in patients with advanced/recurrent gastric cancer: a Japanese Cooperative Study Group trial (group A). Gan To Kagaku Ryoho. 1998; 25:1915–1924. PMID: 9797814.18. Andre T, Louvet C, Ychou M, Gamelin E, Mousseau M, Carola E, et al. Docetaxel-epirubicin as second-line treatment for patients with advanced gastric cancer. Proc Am Soc Clin Oncol. 1999; 18:1062. (abstr).19. Chun JH, Kim HK, Lee JS, Choi JY, Lee HG, Yoon SM, et al. Weekly irinotecan in patients with metastatic gastric cancer failing cisplatin-based chemotherapy. Jpn J Clin Oncol. 2004; 34:8–13. PMID: 15020657.
Article20. Assersohn L, Brown G, Cunningham D, Ward C, Oates J, Waters JS, et al. Phase II study of irinotecan and 5-fluorouracil/leucovorin in patients with primary refractory or relapsed advanced oesophageal and gastric carcinoma. Ann Oncol. 2004; 15:64–69. PMID: 14679122.
Article21. Kim ST, Kang WK, Kang JH, Park KW, Lee J, Lee SH, et al. Salvage chemotherapy with irinotecan, 5-fluorouracil and leucovorin for taxane- and cisplatin-refractory, metastatic gastric cancer. Br J Cancer. 2005; 92:1850–1854. PMID: 15870718.
Article22. Kim DY, Kim JH, Lee SH, Kim TY, Heo DS, Bang YJ, et al. Phase II study of oxaliplatin, 5-fluorouracil and leucovorin in previously platinum-treated patients with advanced gastric cancer. Ann Oncol. 2003; 14:383–387. PMID: 12598342.
Article23. Lee SS, Lee JL, Ryu MH, Chang HM, Kim TW, Kim WK, et al. Combination chemotherpy with capecitabine (X) and cisplatin (P) as a first line treatment of advanced gastric cancer: experience of 246 patients with prognostic factor analysis. Proc ECCO 13. 2005; (submitted).24. Moiseyenko VM, Ajani JA, Tjulandin SA, Majlis A, Constenla M, Boni C, et al. Final results of a randomized controlled phase III trial (TAX 325) comparing docetaxel (T) combined with cisplatin (C) and 5-fluorouracil (F) to CF in patients (pts) with metastatic gastric adenocarcinoma (MGC). Proc Am So Clin Oncol. 2005; 23:308s. (abstr 4002).
Article25. Kang YK, Kim TW, Chang HM, Ryu MH, Yook JH, Oh ST, et al. A phase I/II trial of docetaxel, capecitabine, and cisplatin as a first line chemotherapy for advanced gastric cancer. Proc Am So Clin Oncol. 2004; 22:(abstr 4066).
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Chemotherapy for Metastatic Gastric Cancer
- Chemotherapy for Advanced Gastric Cancer in Elderly Patients
- A New Option for Advanced Gastric Cancer: Docetaxel and Novel Oral Fluoropyrimidine Combination Chemotherapy
- Treatment for unresectable gastric cancer
- Curative Resection of Inoperable, Locally Advanced Gastric Cancer after Neoadjuvant Chemotherapy with Taxotere and Cisplatin