Cancer Res Treat.
2004 Oct;36(5):287-292.
A Phase II Trial of Docetaxel and Ifosfamide for Patients with Platinum-Resistant or Refractory Non-Small Cell Lung Cancer in a Salvage Setting
- Affiliations
-
- 1Division of Hematology-Oncology, Departments of Internal Medicine, College of Medicine, Gyeong-Sang National University, Jinju, Korea.
- 2Department of Internal Medicine, College of Medicine, Seoul National University Bundang Hospital, Seongnam, Korea. jslee@snubh@org
Abstract
- PURPOSE
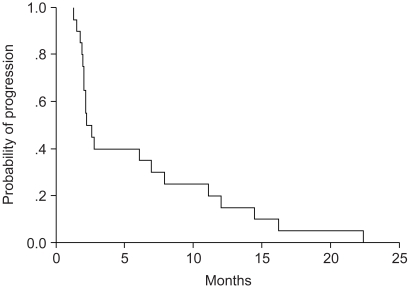
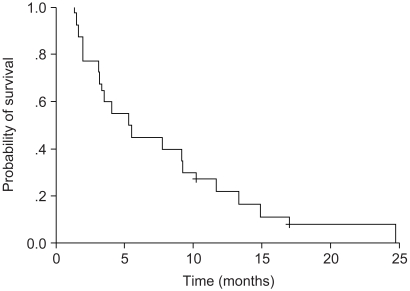
We conducted a phase II study of docetaxel and ifosfamide chemotherapy for patients with platinum- resistant or refractory non-small-cell lung cancer (NSCLC) to evaluate the response and toxicity profiles as a salvage treatment. MATERIALS AND METHODS: Between July 2000 and July 2004, 40 patients who had previously received platinum- based regimen as the first-line or second-line therapy were enrolled in this study. The treatment consisted of a docetaxel 75 mg/m2 intravenous infusion on day 1 and intravenous ifosfamide 3 g/m2 with Mesna(R) uroprotectione on day 1 through 3. This regimen was repeated every 3 weeks. RESULTS: One hundred thirty cycles of treatment were given, with a median of 3 cycles (range: 2~6 cycles). All the patients were evaluable for the response rate and toxicity profile. The major toxicity was myelosuppression. Grade 3~4 neutropenia occurred in 30 patients (75%) during treatment. Febrile neutropenia occurred in 16 patients (40%). Five of 40 patients (12.5%) had a partial response (95% confidence interval, 3.3~21.7%). The median time to disease progression was 2.65 months (range: 2.02~3.20 months), and the median survival was 5.24 months (range: 2.99~7.49 months). CONCLUSION: Salvage chemotherapy with docetaxel and ifosfamide showed a low efficacy and a high proportion of severe neutropenia in patients with platinum-resistant or refractory advanced NSCLC.
MeSH Terms
Figure
Reference
-
1. Bae JM, Won YJ, Jung KW, Suh KA, Yun YH, Shin MH, Ahn YO, Lee DH, Shin HR, Ahn DH, Oh DK, Park JG. Survival of Korean cancer patients diagnosed in 1995; 134 Korean Cancer Registry-affiliated Hospitals. Cancer Res Treat. 2002; 34:319–325.2. Vokes EE, Bitran JD, Vogelzang NJ. Chemotherapy for non-small-cell lung cancer: The continuing challenge. Chest. 1991; 99:1326–1328. PMID: 1645242.3. Grilli R, Oxman AD, Julian JA. Chemotherapy for advanced non-small cell lung cancer: How much benfit is enough? J Clin Oncol. 1993; 11:1866–1872. PMID: 8410111.4. Fossella FV, Rigas J. The use of docetaxel (Taxotere) in patients with advanced non-small cell lung cancer previously treated with platinum-containing chemotherapy regimens. Semin Oncol. 1999; 26:9–12. PMID: 10458204.5. Fossella FV, DeVore R, Kerr RN, Natale RR, Dunphy F, Kalman L, Miller V, Lee JS, Moore M, Gandara D, Karp D, Vokes E, Kris M, Kim Y, Gamza F, Hammershaimb L. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small cell lung cancer previously treated with platinum-containing chemotherapy regimens. The TAX 320 Non-Small Cell Lung Cancer Study Group. J Clin Oncol. 2000; 18:2354–2362. PMID: 10856094.6. Shepherd FA, Dancey J, Ramlau R, Mattson K, Gralla R, O'Rourke M, Levitan N, Gressot L, Vincent M, Burkes R, Coughlin S, Kim Y, Berille J. Prosepctive randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000; 18:2095–2103. PMID: 10811675.7. Sculier JP, Paesmans M, Thiriaux J, Lecomite J, Bureau G, Giner V, Efremidis A, Lafitte JJ, Berchier MC, Alexopoulos CG, Zacharias C, Mommen P, Ninane V, Klastersky J. Phase III randomized trial comparing cisplatin and carboplatin with or without ifosfamide in patients with advanced non-small-cell lung cancer. European Lung Cancer Working Party. J Clin Oncol. 1998; 16:1388–1396. PMID: 9552042.
Article8. Johnson DH. Overview of ifosfamide in small cell and non-small cell lung cancer. Semin Oncol. 1990; 6:87–98.9. Paccagnella A, Favaretto A, Brandes A, Ghiotto C, Fornasiero A, Volpi A, Pappagallo G, Festi G, Cipriani A, Vinante O. Cisplatin, etoposide, and ifosfamide in non-small cell lung carcinoma. A phse II randomized study with cisplatin and etoposide as the control arm. Cancer. 1990; 65:2631–2634. PMID: 2160312.10. Pronk LC, Schrikvers D, Schellens JH, de Brujin EA, Planting AS, Locci-Tonelli D, Groult V, Verweij J, van Oosterom AT. Phase I study on docetaxel and ifosfamide in patients with advanced solid tumors. Br J Cancer. 1998; 77:153–158. PMID: 9459161.
Article11. World Health Organization. The WHO Handbook for reporting the results of Cancer Treatment. 1979. Geneva: World Health Organization.12. Simon R. How large should a phase II trial of a new drug be? Cancer Treat Rep. 1987; 71:1079–1085. PMID: 3315196.13. Kosmas C, Tsavaris N, Mylonakis N, Kalofonos HP. An overview of current results with the gemcitabine and docetaxel combination as initial and salvage chemotherapy regimen in advanced non-small cell lung cancer. Crit Rev Oncol Hematol. 2003; 45:265–275. PMID: 12633839.
Article14. Fossella FV, Lee JS, Shin DM, Calayag M, Huber M, Perez-Soler R, Murphy WK, Lippman S, Benner S, Glisson B. Phase II study of docetaxel for advanced or metastatic platinum-refractory non-small-cell lung cancer. J Clin Oncol. 1995; 13:645–651. PMID: 7884425.
Article15. Gandara DR, Vokes E, Green M, Bonomi P, Devore R, Comis R, Carbone D, Karp D, Belani C. Activity of docetaxel in platinum-treated non-small-cell lung cancer: results of a phase II multicenter trial. J Clin Oncol. 2000; 18:131–135. PMID: 10623703.16. Diaz JF, Andreu JM. Assembly of purified GDP-tubulin into microtubule induced by taxol and taxotere: reversibility, ligand stoichiometry, and completition. Biochemistry. 1993; 32:2747–2755. PMID: 8096151.17. Haldar S, Basu A, Croce C. Bcl2 is the guardian of microtubule integrity. Cancer Res. 1997; 57:229–233. PMID: 9000560.18. Cerny T, Kaplan S, Pavlidis N, Schoffski P, Epelbaum R, van Meerbeek J, Wanders J, Franklin HR, Kaye S. Docetaxel (Taxotere) is an active in non-small cell lung cancer: a Phase II trial of the EORTC Early Clinical Trials Group (ECTG). Br J Cancer. 1994; 70:384–387. PMID: 7914429.19. Francis PA, Rigas JR, Kris MG, Pisters KM, Orazem JP, Woolley KJ, Heelan RT. Phase II Trial of docetaxel in patients with stage III and IV non-small-cell lung cancer. J Clin Oncol. 1994; 12:1232–1237. PMID: 7911159.
Article20. Berger DP, Fiebig H, Winterhalter BR, Wallbrecher E, Henss H. Preclinical phase II study of ifosfamide in human tumor xenografts in vivo. Cancer chemother Pharmacol. 1990; 26:S7–S11. PMID: 2347054.21. Drings P, Buchholz E, Manegold C. Ifosfamide and docetaxel in non-small cell lung cancer. Semin Oncol. 1998; 25(1 Suppl 2):29–37. PMID: 9535209.22. Sommer K, Peters SO, Robins IH. A preclinical model for experiemental chemotherapy of human head and neck cancer. Int J Oncol. 2001; 18:1145–1149. PMID: 11351243.23. Krege S, Rembrink V, Borgermann C, Otto T, Rubben H. Docetaxel and ifosfamide as second-line treatment for patients with advanced or metastatic urothelial cancer after failure of platinum chemotherapy: a phase 2 study. J Urol. 2001; 165:67–71. PMID: 11125366.24. Kunitoh H, Akiyama Y, Kusaba H, Yamamoto N, Sekine I, Ohe Y, Kubota K, Tamura T, Shinkai T, Kodama T, Goto K, Niho S, Nishiwaki Y, Saiko N. A phase I/II trial of cisplatin, docetaxel and ifosfamide in advanced or recurrent non-small cell lung cancer. Lung Cancer. 2001; 33:259–265. PMID: 11551421.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Phase I Clinical Trial of Paclitaxel Plus Ifosfamide for the Patients with Refractory Ovarian Cancer
- Clinical Efficacy of Ifosfamide-Based Regimen in Refractory of Relapsed Ovarian Cancer
- Cell Death Induction Mechanism of Non-small Cell Lung Cancer Cell Line, NCI-H1703 by Docetaxel
- Phase II Study of Ifosfamide, Etoposide and Cisplatin(IEP) Chemotherapy for Advanced Non-Small Cell Lung Cancer
- Phase II Study of S-1 Plus Either Irinotecan or Docetaxel for Non-small Cell Lung Cancer Patients Treated with More Than Three Lines of Treatment