Yonsei Med J.
2013 Nov;54(6):1430-1437. 10.3349/ymj.2013.54.6.1430.
Effects of Diet-Induced Mild Obesity on Airway Hyperreactivity and Lung Inflammation in Mice
- Affiliations
-
- 1Department of Pediatrics, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea. jy7.shim@samsung.com
- 2Institute of Medical Research, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 3Department of Endocrinology and Metabolism, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 1798140
- DOI: http://doi.org/10.3349/ymj.2013.54.6.1430
Abstract
- PURPOSE
Obesity has been suggested to be linked to asthma. However, it is not yet known whether obesity directly leads to airway hyperreactivity (AHR) or obesity-induced airway inflammation associated with asthma. We investigated obesity-related changes in adipokines, AHR, and lung inflammation in a murine model of asthma and obesity.
MATERIALS AND METHODS
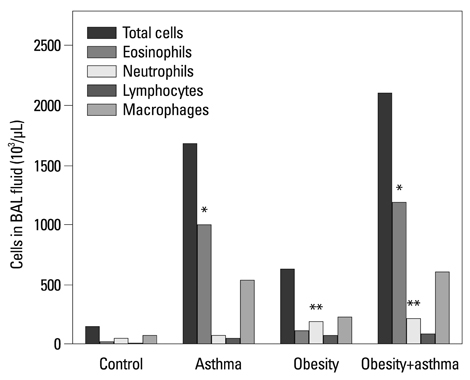
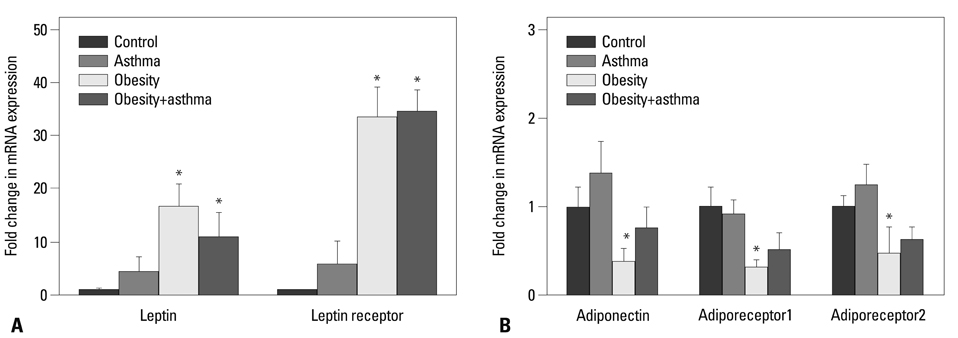
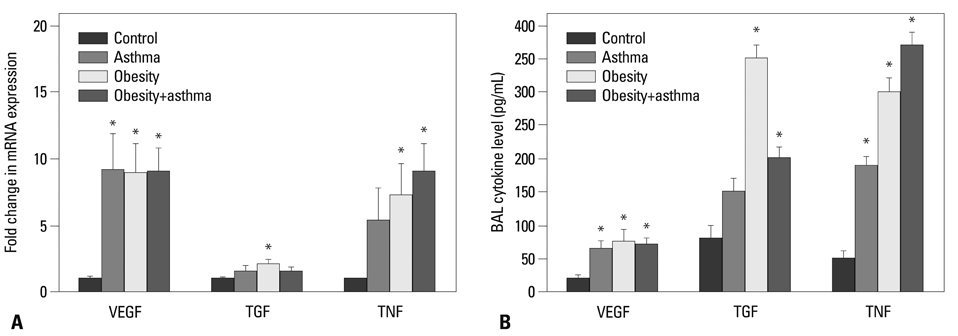
We developed mouse models of chronic asthma via ovalbumin (OVA)-challenge and of obesity by feeding a high-fat diet, and then performed the methacholine bronchial provocation test, and real-time PCR for leptin, leptin receptor, adiponectin, adiponectin receptor (adipor1 and 2), vascular endothelial growth factor (VEGF), transforming growth factor (TGF) beta, and tumor necrosis factor (TNF) alpha in lung tissue. We also measured cell counts in bronchoalveolar lavage fluid.
RESULTS
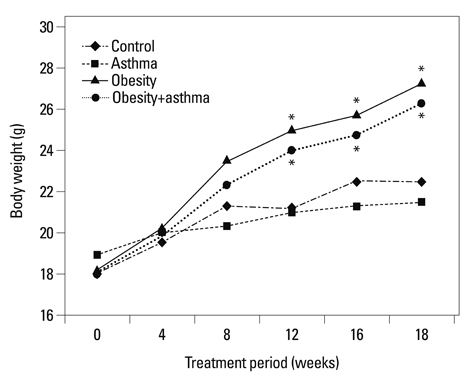
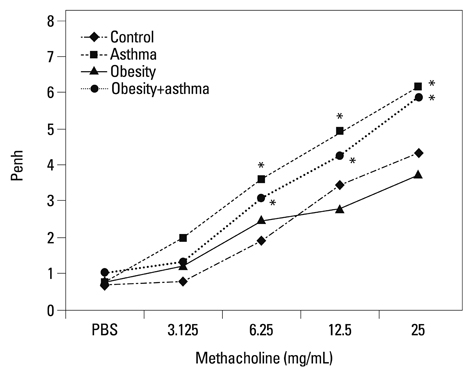
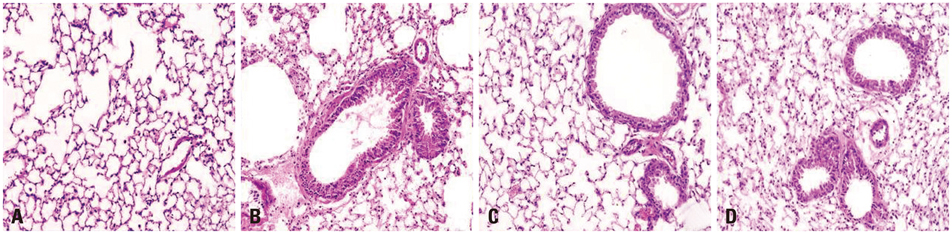
Both obese and lean mice chronically exposed to OVA developed eosinophilic lung inflammation and AHR to methacholine. However, obese mice without OVA challenge did not develop AHR or eosinophilic inflammation in lung tissue. In obese mice, lung mRNA expressions of leptin, leptin receptor, VEGF, TGF, and TNF were enhanced, and adipor1 and 2 expressions were decreased compared to mice in the control group. On the other hand, there were no differences between obese mice with or without OVA challenge.
CONCLUSION
Diet-induced mild obesity may not augment AHR or eosinophilic lung inflammation in asthma.
Keyword
MeSH Terms
-
Animals
Asthma/physiopathology
Bronchial Hyperreactivity/*physiopathology
Bronchoalveolar Lavage Fluid/chemistry
Dietary Fats/adverse effects
Mice
Obesity/*etiology/*physiopathology
Pneumonia/*physiopathology
Transforming Growth Factors/metabolism
Tumor Necrosis Factor-alpha/metabolism
Vascular Endothelial Growth Factor A/metabolism
Dietary Fats
Transforming Growth Factors
Tumor Necrosis Factor-alpha
Vascular Endothelial Growth Factor A
Figure
Reference
-
1. Koenig SM. Pulmonary complications of obesity. Am J Med Sci. 2001; 321:249–279.
Article2. Stunkard AJ. Current views on obesity. Am J Med. 1996; 100:230–236.
Article3. Ford ES. The epidemiology of obesity and asthma. J Allergy Clin Immunol. 2005; 115:897–909.
Article4. Taylor B, Mannino D, Brown C, Crocker D, Twum-Baah N, Holguin F. Body mass index and asthma severity in the National Asthma Survey. Thorax. 2008; 63:14–20.
Article5. Shore SA. Obesity and asthma: possible mechanisms. J Allergy Clin Immunol. 2008; 121:1087–1093.
Article6. Litonjua AA, Gold DR. Asthma and obesity: common early-life influences in the inception of disease. J Allergy Clin Immunol. 2008; 121:1075–1084.
Article7. Husemoen LL, Glümer C, Lau C, Pisinger C, Mørch LS, Linneberg A. Association of obesity and insulin resistance with asthma and aeroallergen sensitization. Allergy. 2008; 63:575–582.
Article8. Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000; 21:697–738.
Article9. Hausman GJ, Richardson RL. Adipose tissue angiogenesis. J Anim Sci. 2004; 82:925–934.10. Ryu SL, Shim JW, Kim DS, Jung HL, Park MS, Park SH, et al. Expression of peroxisome proliferator-activated receptor (PPAR)-α and PPAR-γ in the lung tissue of obese mice and the effect of rosiglitazone on proinflammatory cytokine expressions in the lung tissue. Korean J Pediatr. 2013; 56:151–158.
Article11. Zhao AZ, Shinohara MM, Huang D, Shimizu M, Eldar-Finkelman H, Krebs EG, et al. Leptin induces insulin-like signaling that antagonizes cAMP elevation by glucagon in hepatocytes. J Biol Chem. 2000; 275:11348–11354.
Article12. Johnston RA, Theman TA, Lu FL, Terry RD, Williams ES, Shore SA. Diet-induced obesity causes innate airway hyperresponsiveness to methacholine and enhances ozone-induced pulmonary inflammation. J Appl Physiol. 2008; 104:1727–1735.
Article13. Lu FL, Johnston RA, Flynt L, Theman TA, Terry RD, Schwartzman IN, et al. Increased pulmonary responses to acute ozone exposure in obese db/db mice. Am J Physiol Lung Cell Mol Physiol. 2006; 290:L856–L865.14. Shore SA. Obesity, airway hyperresponsiveness, and inflammation. J Appl Physiol. 2010; 108:735–743.
Article15. Johnston RA, Theman TA, Shore SA. Augmented responses to ozone in obese carboxypeptidase E-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2006; 290:R126–R133.
Article16. Dietze J, Böcking C, Heverhagen JT, Voelker MN, Renz H. Obesity lowers the threshold of allergic sensitization and augments airway eosinophilia in a mouse model of asthma. Allergy. 2012; 67:1519–1529.
Article17. Calixto MC, Lintomen L, Schenka A, Saad MJ, Zanesco A, Antunes E. Obesity enhances eosinophilic inflammation in a murine model of allergic asthma. Br J Pharmacol. 2010; 159:617–625.
Article18. Shore SA, Schwartzman IN, Mellema MS, Flynt L, Imrich A, Johnston RA. Effect of leptin on allergic airway responses in mice. J Allergy Clin Immunol. 2005; 115:103–109.
Article19. Fantuzzi G. Adiponectin and inflammation: consensus and controversy. J Allergy Clin Immunol. 2008; 121:326–330.
Article20. Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003; 423:762–769.
Article21. Shore SA, Terry RD, Flynt L, Xu A, Hug C. Adiponectin attenuates allergen-induced airway inflammation and hyperresponsiveness in mice. J Allergy Clin Immunol. 2006; 118:389–395.
Article22. Silha JV, Krsek M, Sucharda P, Murphy LJ. Angiogenic factors are elevated in overweight and obese individuals. Int J Obes (Lond). 2005; 29:1308–1314.
Article23. Shin JH, Kim JH, Lee WY, Shim JY. The expression of adiponec tin receptors and the effects of adiponectin and leptin on airway smooth muscle cells. Yonsei Med J. 2008; 49:804–810.
Article24. Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993; 259:87–91.
Article25. Thomas PS, Yates DH, Barnes PJ. Tumor necrosis factor-alpha increases airway responsiveness and sputum neutrophilia in normal human subjects. Am J Respir Crit Care Med. 1995; 152:76–80.
Article26. Vignola AM, Mirabella F, Costanzo G, Di Giorgi R, Gjomarkaj M, Bellia V, et al. Airway remodeling in asthma. Chest. 2003; 123:3 Suppl. 417S–422S.
Article27. Redington AE, Madden J, Frew AJ, Djukanovic R, Roche WR, Holgate ST, et al. Transforming growth factor-beta 1 in asthma. Measurement in bronchoalveolar lavage fluid. Am J Respir Crit Care Med. 1997; 156(2 Pt 1):642–647.28. Shin JH, Shim JW, Kim DS, Shim JY. TGF-beta effects on airway smooth muscle cell proliferation, VEGF release and signal transduction pathways. Respirology. 2009; 14:347–353.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- CD4+ T Helper Cells Engineered to Produce IL-10 Reverse Allergen-induced Airway Hyperreactivity and Inflammation
- Effect of the anti-IL-17 antibody on allergic inflammation in an obesity-related asthma model
- Effects of CpG Oligodeoxynucleotide on Airway Inflammation after Topical Application of and Airway Challenge with Dermatophagoides farinae in NC/Nga Mice
- An Intratracheal Challenge Murine Model of Asthma: Can Bronchial Inflammation Affect the Nose?
- The Inhibitory Effect of Quercetin on Adipose Tissue Inflammation in Mice Fed on a High-fat Diet