J Korean Med Sci.
2010 Jul;25(7):1017-1023. 10.3346/jkms.2010.25.7.1017.
Ectopic Over-expression of Oncogene Pim-2 Induce Malignant Transformation of Nontumorous Human Liver Cell Line L02
- Affiliations
-
- 1Department of Hepatobiliary Surgery, the Second Affiliated Hospital of Chongqing Medical University, Chongqing, P.R. China. gongjianping11@126.com
- 2Transplantation Immunity Laboratory, West China Hospital of Sichuan University, Chengdu, P.R. China.
- KMID: 1792950
- DOI: http://doi.org/10.3346/jkms.2010.25.7.1017
Abstract
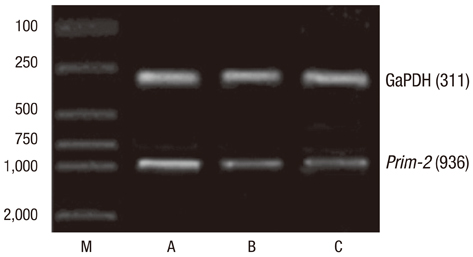
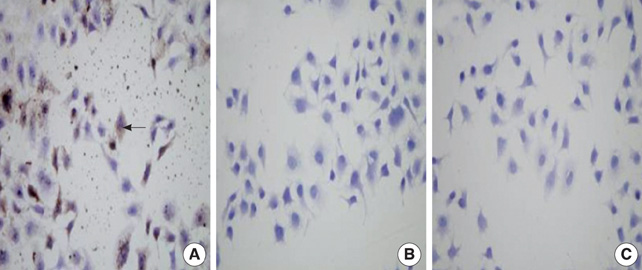
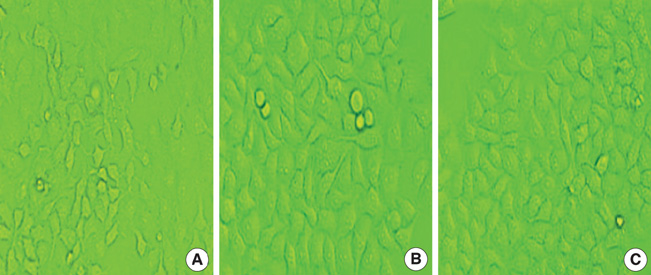
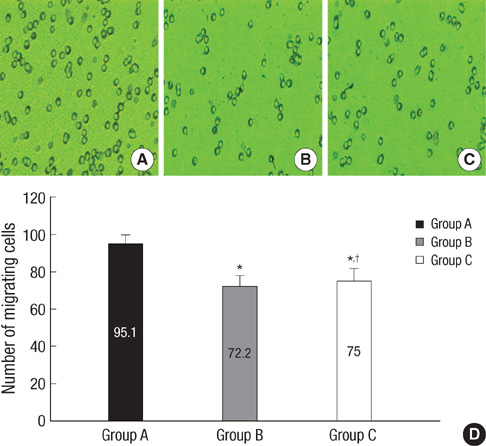
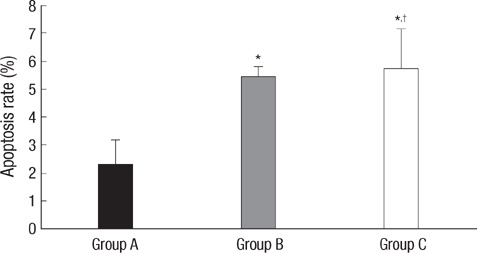
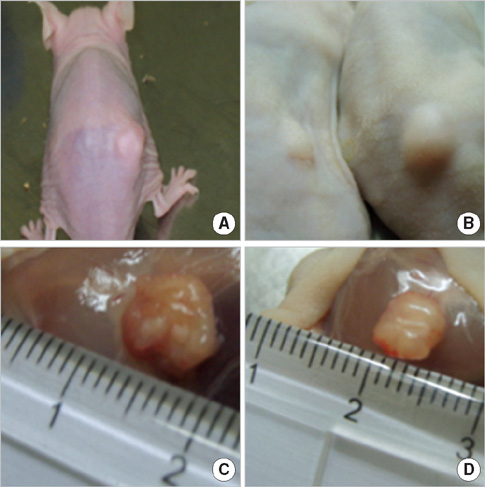
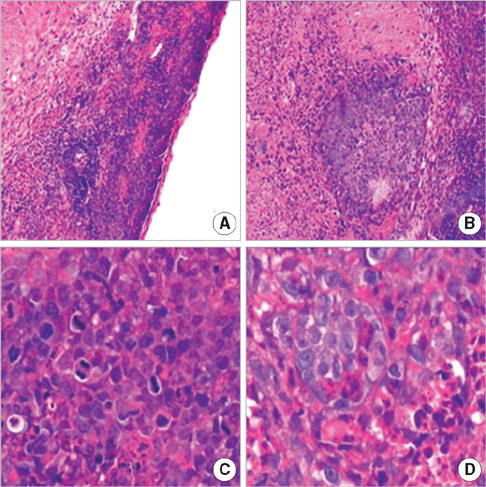
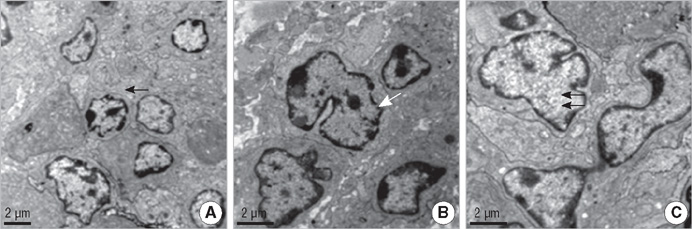
- In order to prove that ectopic over-expression of Pim-2 could induce malignant transformation of human liver cell line L02, three groups of cells were set up including human liver cell line L02 (L02), L02 cells transfected with Pim-2 gene (L02/Pim-2) and L02 cells transfected with empty-vector (L02/Vector). Pim-2 expression levels were detected. The morphology, proliferation level, apoptosis rate and migration ability of the cells were detected respectively. Then the cells were subcutaneously inoculated into athymic mice and the microstructures of the neoplasm were observed. Compared with the controls, Pim-2 expression levels were significantly higher in L02/Pim-2 cells (P<0.05), and their morphology had obvious malignant changes. They also showed a significantly increased proliferation rate (P<0.05) and migration capacity (P<0.05), as well as a significantly decreased apoptosis rate (P<0.05). Only the athymic mice inoculated with L02/Pim-2 cells could generate neoplasm, and the morphology of the neoplasm coincided with that of the hepatoma. The results manifest that ectopic Pim-2 gene could be stably expressed in L02/Pim-2 cells. Both the morphological and biological changes of L02/Pim-2 cells demonstrate the trend of malignant transformation. L02/Pim-2 cells could generate hepatoma in athymic mice. In conclusion, Pim-2 could induce malignant transformation of human liver cell line L02.
Keyword
MeSH Terms
-
Animals
Apoptosis
Cell Line
Cell Movement
Cell Proliferation
*Cell Transformation, Neoplastic
Humans
Liver/pathology/physiology
*Liver Neoplasms/genetics/pathology
Mice
Mice, Nude
Neoplasm Transplantation
*Oncogenes
Protein-Serine-Threonine Kinases/genetics/*metabolism
Proto-Oncogene Proteins/genetics/*metabolism
Figure
Reference
-
1. Fox CJ, Hammerman PS, Cinalli RM. The serine/threonine kinase Pim-2 is a transcriptionally regulated apoptotic inhibitor. Genes Dev. 2003. 17:1841–1854.
Article2. Chen WW, Chan DC, Donald C, Lilly MB, Kraft AS. Pim family kinases enhance tumor growth of prostate cancer cells. Mol Cancer Res. 2005. 3:443–451.
Article3. Adam M, Pogacic V, Bendit M, Chappuis R, Nawijn MC, Duyster J, Fox CJ, Thompson CB, Cools J, Schwaller J. Targeting Pim kinases impairs survival of hematopoietic cells transformed by kinase inhibitor-sensitive and kinase inhibitor resistant forms of Fms-like tyrosine kinase 3 and BCR/ABL. Cancer Res. 2006. 66:3828–3835.4. Mahadevan D, Spier C, Della Croce K, Miller S, George B, Riley C, Warner S, Grogan TM, Miller TP. Transcript profiling in peripheral T-cell lymphoma, not otherwise specified, and diffuse large B-cell lymphoma identifies distinct tumor profile signatures. Mol Cancer Ther. 2005. 4:1867–1879.
Article5. Cohen AM, Grinblat B, Bessler H, Kristt D, Kremer A, Schwartz A, Halperin M, Shalom S, Merkel D, Don J. Increased expression of the hPim-2 gene in human chronic lymphocytic leukemia and non-Hodgkin lymphoma. Leuk Lymphoma. 2004. 45:951–955.6. Chen XP, Losman JA, Cowan S, Donahue E, Fay S, Vuong BQ, Nawijn MC, Capece D, Cohan VL, Rothman P. Pim serine/threonine kinases regulate the stability of Socs-1 protein. Proc Natl Acad Sci USA. 2002. 99:2175–2180.
Article7. Gong J, Wang J, Ren K, Liu C, Li B, Shi Y. Serine/threonine kinase Pim-2 promotes liver tumorigenesis induction through mediating survival and preventing apoptosis of liver cell. J Surg Res. 2009. 153:17–22.
Article8. Guo F, Sigua C, Bali P, George P, Fiskus W, Scuto A, Annavarapu S, Mouttaki A, Sondarva G, Wei S, Wu J, Djeu J, Bhalla K. Mechanistic role of heat shock protein 70 in Bcr-Abl-mediated resistance to apoptosis in human acute leukemia cells. Blood. 2005. 105:1246–1255.
Article9. Ayala GE, Dai H, Ittmann M, Li R, Powell M, Frolov A, Wheeler TM, Thompson TC, Rowley D. Growth and survival mechanisms associated with perineural invasion in prostate cancer. Cancer Res. 2004. 64:6082–6090.
Article10. Dai JM, Zhang SQ, Zhang W, Lin RX, Ji ZZ, Wang SQ. Antisense oligodeoxynucleotides targeting the serine/threonine kinase Pim-2 inhibited proliferation of DU-145 cells. Acta Pharmacol Sin. 2005. 26:364–368.
Article11. White E. The pims and outs of survival signaling: role for the Pim-2 protein kinase in the suppression of apoptosis by cytokines. Genes Dev. 2003. 17:1813–1816.
Article12. Macdonald A, Campbell DG, Toth R, McLauchlan H, Hastie CJ, Arthur JS. Pim kinases phosphorylate multiple sites on Bad and promote 14-3-3 binding and dissociation from Bcl-XL. BMC Cell Biol. 2006. 7:1.13. Peng C, Knebel A, Morrice NA, Li X, Barringer K, Li J, Jakes S, Werneburg B, Wang L. Pim kinase substrate identification and specificity. J Biochem. 2007. 141:353–362.
Article14. Wang Y, Lee AT, Ma JZ, Wang J, Ren J, Yang Y, Tantoso E, Li KB, Ooi LL, Tan P, Lee CG. Profiling microRNA expression in hepatocellular carcinoma reveals microRNA-224 up-regulation and apoptosis inhibitor-5 as a microRNA-224-specific target. J Biol Chem. 2008. 283:13205–13215.
Article15. Hammerman PS, Fox CJ, Cinalli RM, Xu A, Wagner JD, Lindsten T, Thompson CB. Lymphocyte transformation by Pim-2 is dependent on nuclear factor-kappaB activation. Cancer Res. 2004. 64:8341–8348.16. Lin A, Karin M. NF-kappa B in cancer: a marked target. Semin Cancer Biol. 2003. 13:107–114.17. Karin M, Lin A. NF-kappaB at the crossroads of life and death. Nat Immunol. 2002. 3:221–227.18. Ren K, Zhang W, Shi Y, Gong J. Pim-2 activates API-5 to inhibit the apoptosis of hepatocellular carcinoma cells through NF-kappaB pathway. Pathol Oncol Res. 2009. 10. 12. DOI 10.1007/s12253-009-9215-4.19. Datta SR, Ranger AM, Lin MZ, Sturgill JF, Ma YC, Cowan CW, Dikkes P, Korsmeyer SJ, Greenberg ME. Survival factor-mediated BAD phosphorylation raises the mitochondrial threshold for apoptosis. Dev Cell. 2002. 3:631–643.
Article20. Pelengaris S, Khan M, Evan G. c-MYC: more than just a matter of life and death. Nat Rev Cancer. 2002. 2:764–776.
Article21. Dai H, Li R, Wheeler T, Diaz de Vivar A, Frolov A, Tahir S, Agoulnik I, Thompson T, Rowley D, Ayala G. Pim-2 upregulation: biological implications associated with disease progression and perineural invasion in prostate cancer. Prostate. 2005. 65:276–286.22. Aho TL, Sandholm J, Peltola KJ, Mankonen HP, Lilly M, Koskinen PJ. Pim-1 kinase promotes inactivation of the pro-apoptotic BAD protein by phosphorylating it on the Ser112 gatekeeper site. FEBS Lett. 2004. 571:43–49.23. Leverson JD, Koskinen PJ, Orrico FC, Rainio EM, Jalkanen KJ, Dash AB, Eisenman RN, Ness SA. Pim-1 kinase and pl00 cooperate to enhance c-Myb activity. Mol Cell. 1998. 2:417–425.24. Peng C, Knebel A, Morrice NA, Li X, Barringer K, Li J, Jakes S, Werneburg B, Wang L. Pim kinase substrate identification and specificity. J Biochem. 2007. 141:353–362.
Article25. Fujii C, Nakamoto Y, Lu P, Tsuneyama K, Popivanova BK, Kaneko S, Mukaida N. Aberrant expression of serine/threonine kinase Pim-3 in hepatocellular carcinoma development and its role in the proliferation of human hepatoma cell lines. Int J Cancer. 2005. 114:209–218.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pim-1: A serine/threonine kinase with a role in cell survival, proliferation, differentiation and tumorigenesis
- The Expression of ras Oncogene in Benign and Malignant Lesions of Breast
- PIM Kinase as an Executional Target in Cancer
- The differentiation of human multipotent adult progenitor cells into hepatocyte-like cells induced by coculture with human hepatocyte line L02
- Relationship between Proliferative Activity and Expression of HBcAg and p53 Protein in Hepatocellular Carcinoma and Surrounding Nontumorous Liver