Korean J Gastroenterol.
2009 Jun;53(6):361-368. 10.4166/kjg.2009.53.6.361.
Analysis of Gene Expression in Primary Hepatocellular Carcinoma Using Differentially Displayed Reverse Transcriptase Polymerase Chain Reaction
- Affiliations
-
- 1Integrative Research Support Center, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 2WHO Collaborating Center of Viral Hepatitis, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 3Department of Internal Medicine, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- KMID: 1792753
- DOI: http://doi.org/10.4166/kjg.2009.53.6.361
Abstract
-
BACKGROUND/AIMS: The investigation of a specific tumor marker for hepatocellular carcinoma (HCC) is needed to examine the carcinogenesis and to select the patients for treatment options. The aim of this study was to find the genes related to HCC. We also examined the expression level of these genes in cancer cell lines and tissue specimens.
METHODS
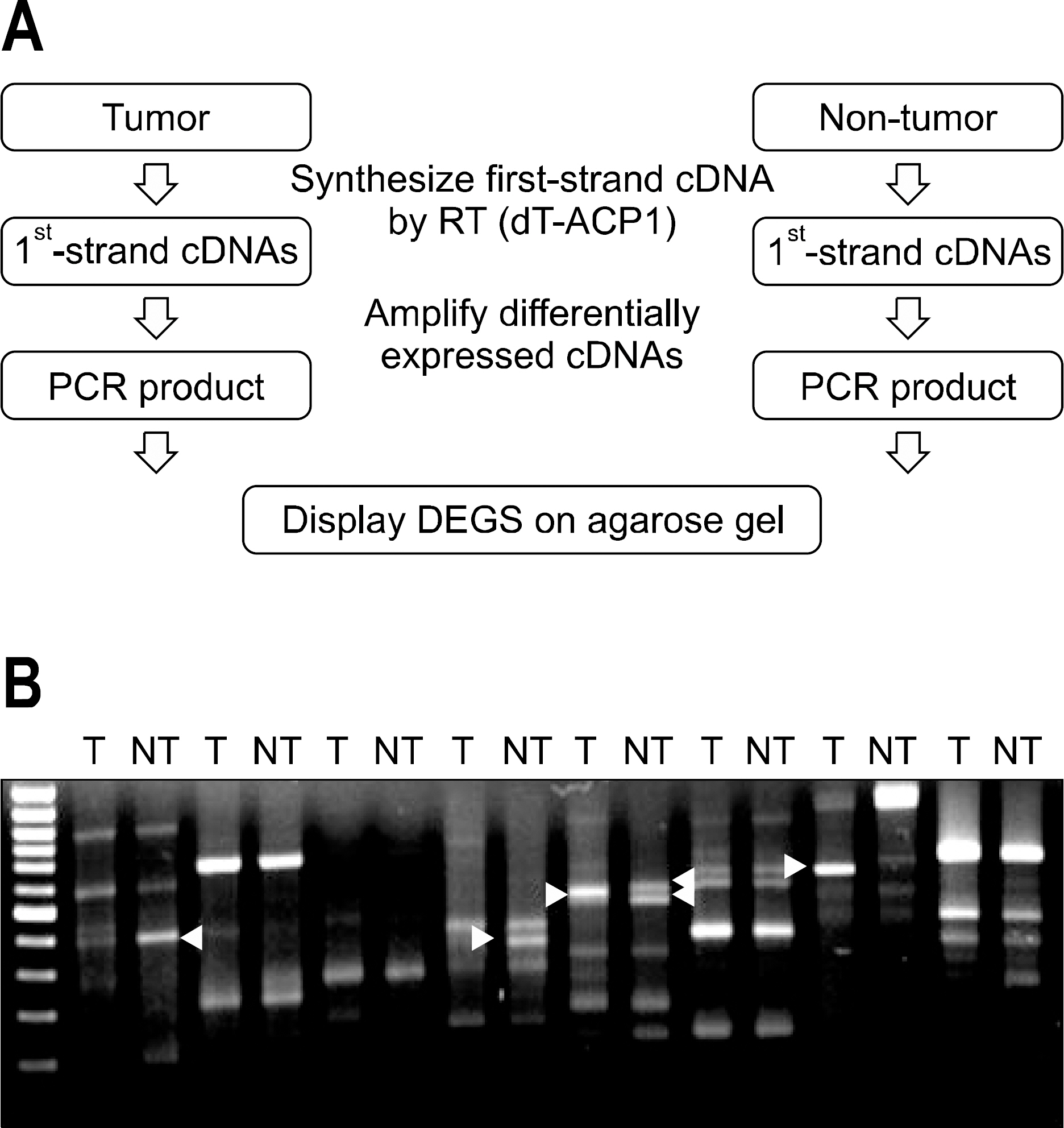
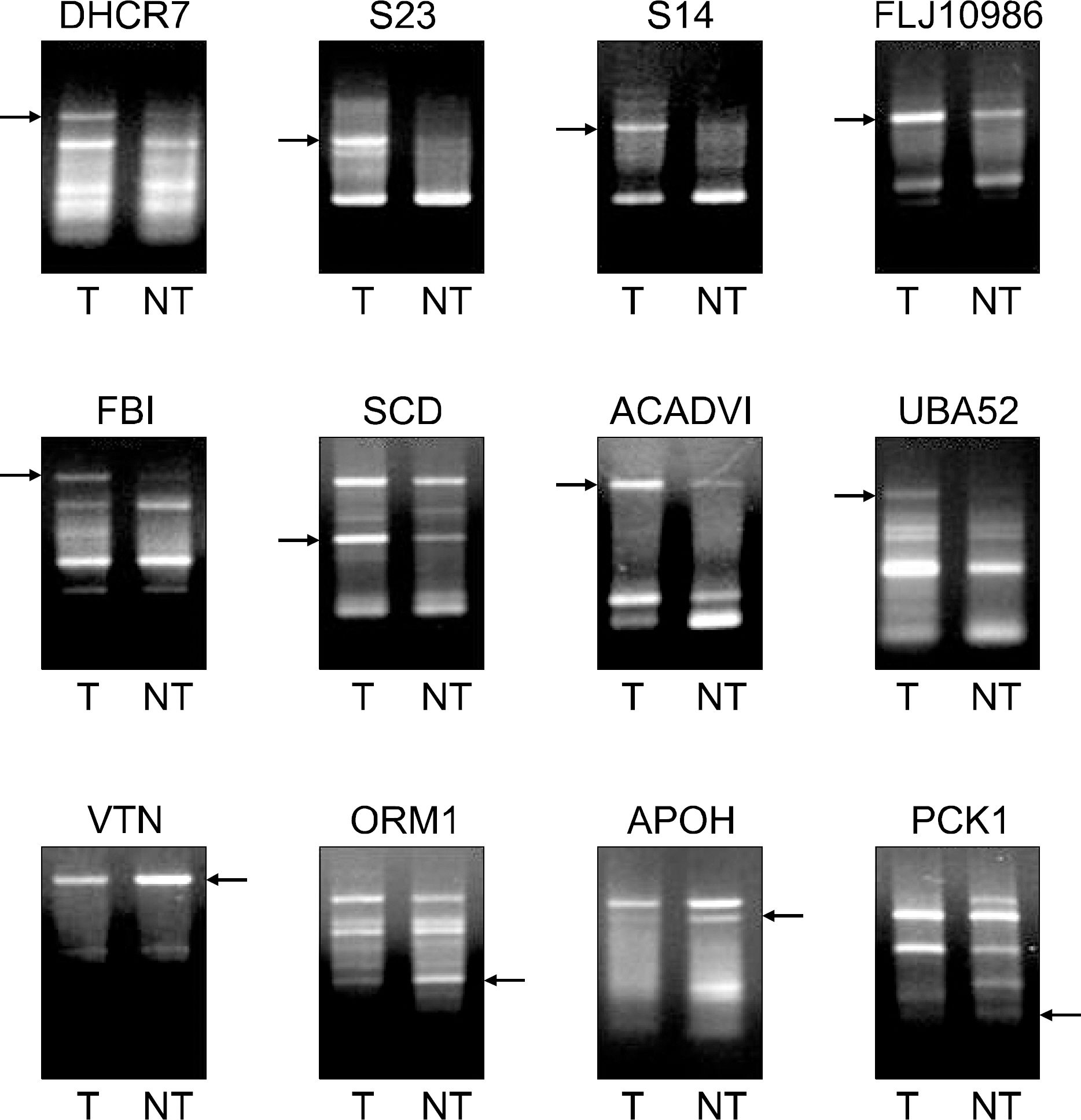
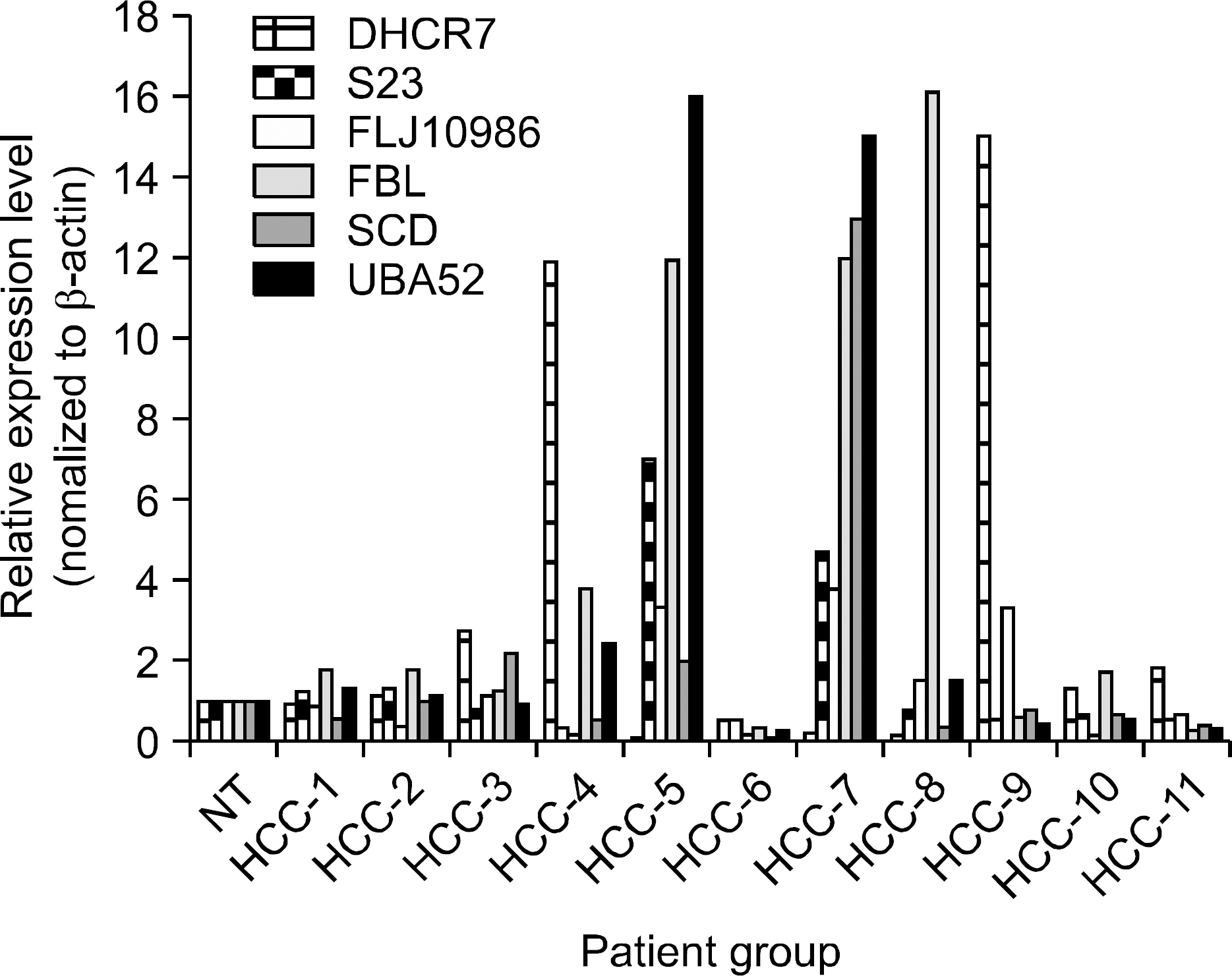
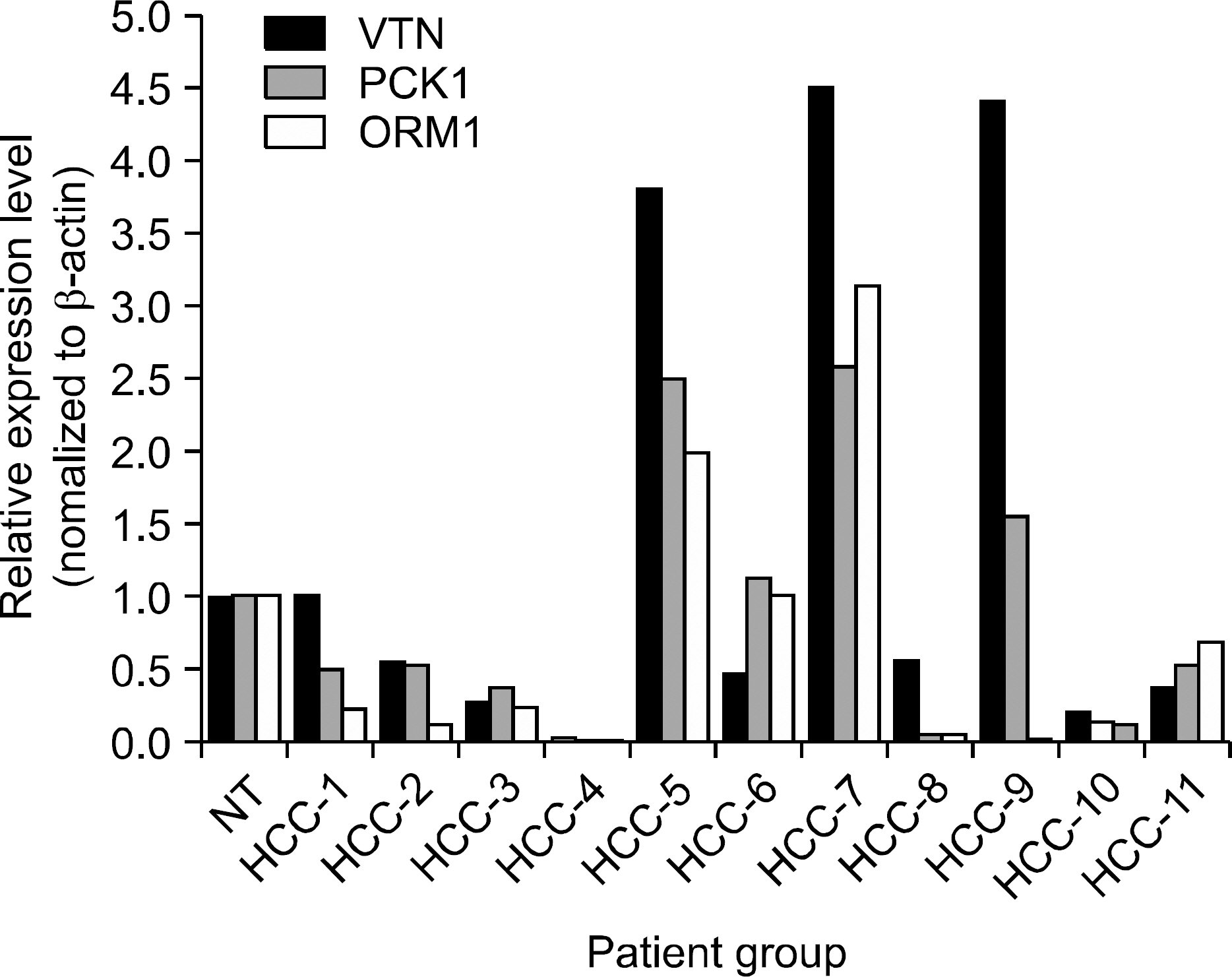
Three pairs of HCC tissue and non-neoplastic hepatic tissue around the HCC were collected from three patients who underwent resection for HCC. Differential display reverse transcriptase-PCR (DD RT-PCR) using GeneFishing (TM) PCR was used to detect the differences in the gene expression between in HCC tissue and non-neoplatic tissue. Up- or down-regulated genes in HCC tissue were identified through BLAST searches after cloning and sequencing assays. Real-time RT-PCR assay was employed to detect the expression rate in 11 HCC tissues and human cancer cell lines.
RESULTS
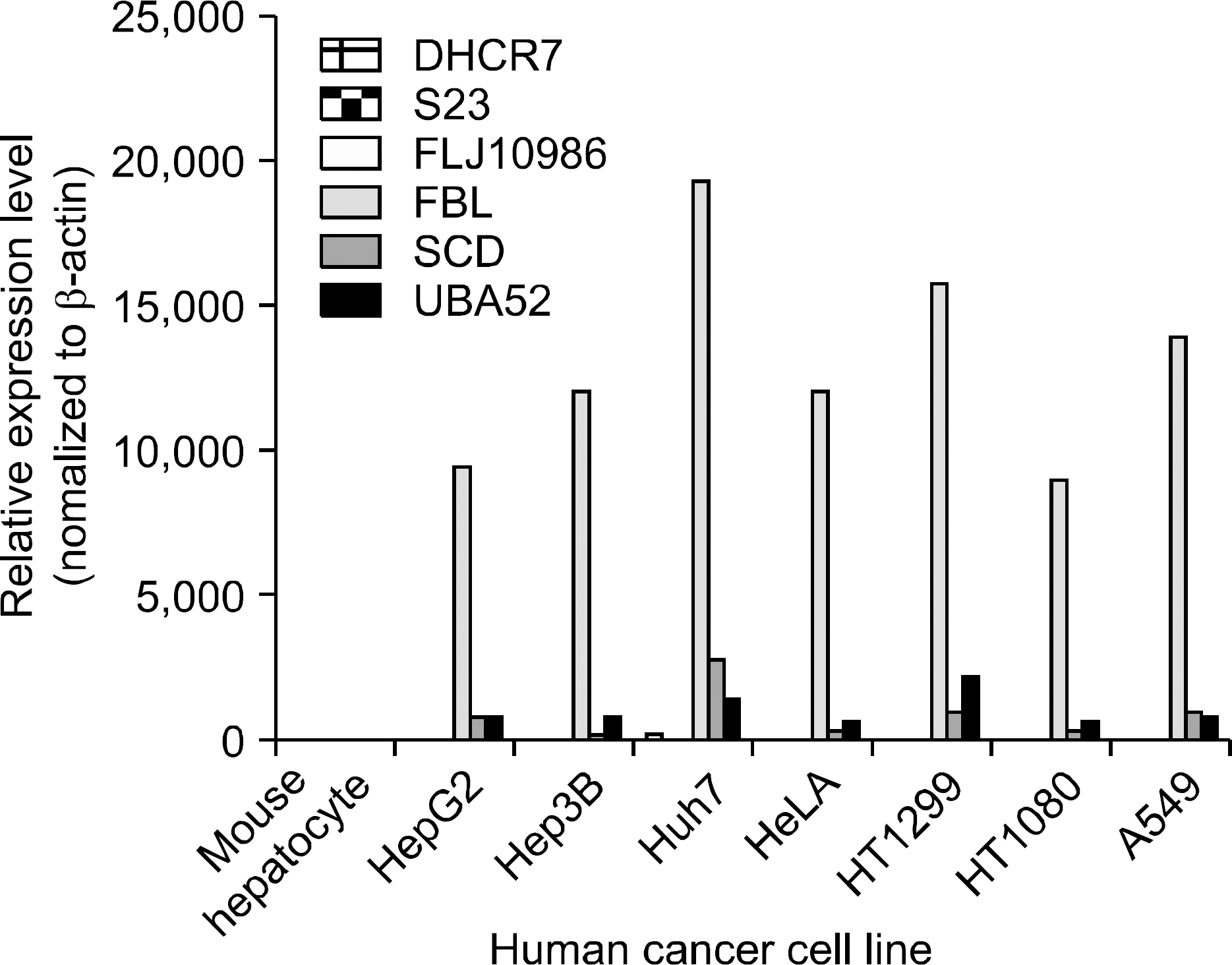
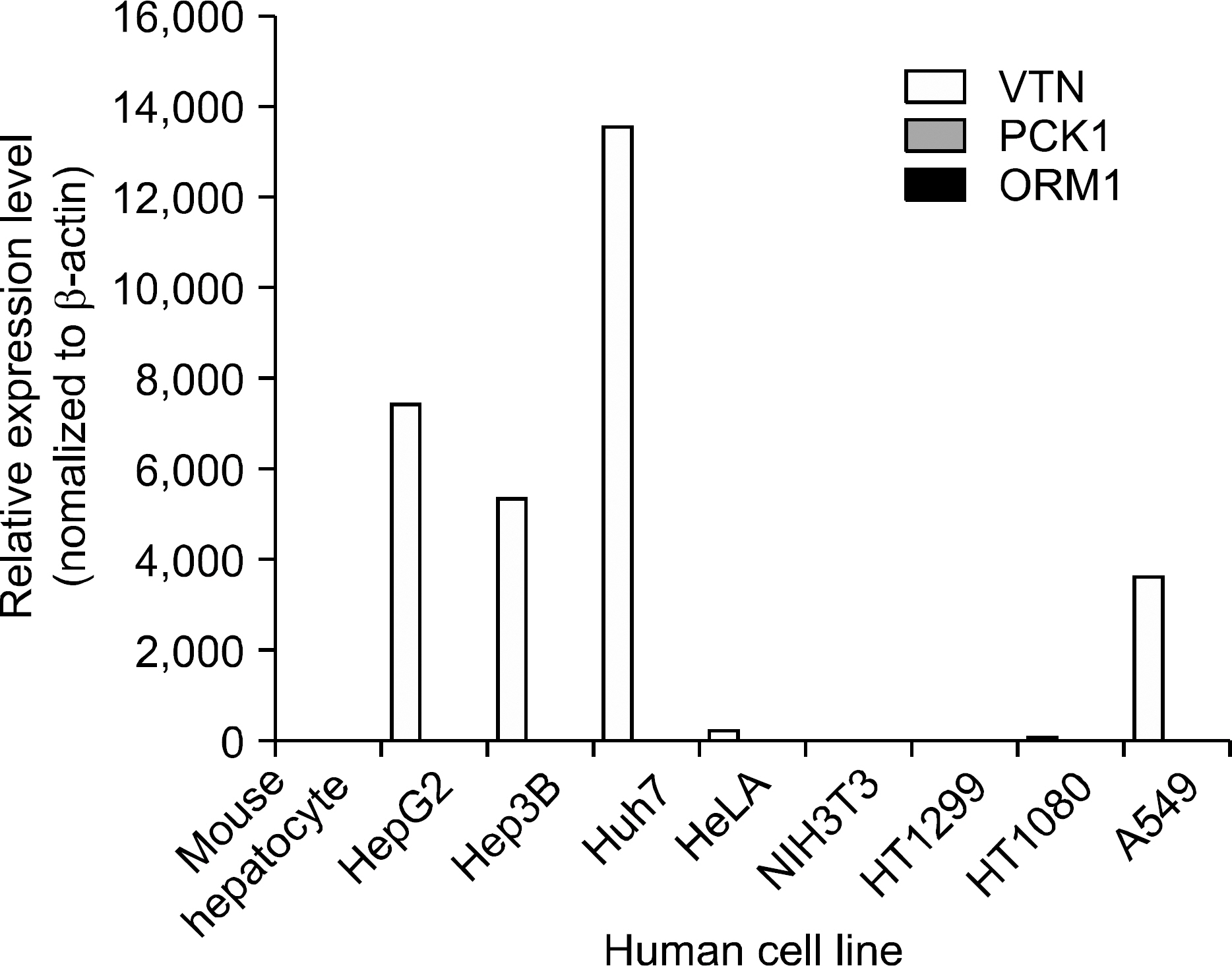
Differentially expressed 21 genes were identified, and they were classified as genes involved in protein metabolism, ubiquitin-dependent protein catabolism, carbohydrate metabolism, lipid metabolism, DNA repair, and inflammatory response.
CONCLUSIONS
We identified differentially expressed genes in HCC, and these genes may play an important role in the study of hepatocarcinogenesis, development of biomarker, and target therapy for HCC.
Keyword
MeSH Terms
-
Carcinoma, Hepatocellular/diagnosis/genetics/*metabolism
Cell Line, Tumor
Down-Regulation
Gene Expression Regulation, Neoplastic
Humans
Liver Neoplasms/diagnosis/genetics/*metabolism
Reverse Transcriptase Polymerase Chain Reaction
Sequence Analysis, DNA
Tumor Markers, Biological/genetics/*metabolism
Up-Regulation
Figure
Reference
-
1. Park JW, Kim CM. Epidemiology of hepatocellular carcinoma in Korea. Korean J Hepatol. 2005; 11:303–310.2. Kaino M. Alterations in the tumor suppressor genes p53, RB, p16/MTS1, and p15/MTS2 in human pancreatic cancer and hepatoma cell lines. J Gastroenterol. 1997; 32:40–46.3. Ashida K, Kishimoto Y, Nakamoto K, et al. Loss of heterozygosity of the retinoblastoma gene in liver cirrhosis accom-panying hepatocellular carcinoma. J Cancer Res Clin Oncol. 1997; 123:489–495.
Article4. Kondoh N, Wakatsuki T, Ryo A, et al. Identification and characterization of genes associated with human hepatocellular carcinogenesis. Cancer Res. 1999; 59:4990–4996.5. Song JY, Choi JH, Lee KJ, et al. Identification of the gene associated with hepatocellular carcinoma using differential gene expresion. Korean J Hepatol. 2001; 7:265–272.6. Cui XS, Shin MR, Lee KA, Kim NH. Identification of differentially expressed genes in murine embryos at the blastocyst stage using annealing control primer system. Mol Reprod Dev. 2005; 70:278–287.
Article7. Okabe H, Satoh S, Kato T, et al. Genomewide analysis of gene expression in human hepatocellular carcinomas using cDNA microarray: identification of genes involved in viral carcinogenesis and tumor progression. Cancer Res. 2001; 61:2129–2137.8. Jia HL, Ye QH, Qin LX, et al. Gene expression profiling re-veals potential biomarkers of human hepatocellular carcinoma. Clin Cancer Res. 2007; 13:1133–1139.
Article9. Villanueva A, Newell P, Chiang DY, Friedman SL, Llovet JM. Genomics and signaling pathways in hepatocellular carcinoma. Semin Liver Dis. 2007; 27:55–76.
Article10. Wang ZX, Wang HY, Wu MC. Identification and characterization of a novel human hepatocellular carcinoma-associated gene. Br J Cancer. 2001; 85:1162–1167.
Article11. Nomura F, Yaguchi M, Togawa A, et al. Enhancement of poly-adenosine diphosphate- ribosylation in human hepatocellular carcinoma. J Gastroenterol Hepatol. 2000; 15:529–535.12. Yu SW, Andrabi SA, Wang H, Kim NS, Poirier GG, Dawson TM, et al. Apoptosis-inducing factor mediates poly(ADP-ri-bose) (PAR) polymer-induced cell death. PNAS. 2006; 103:18314–18319.
Article13. Westra R, Jansen TL, Gouw AS, de Jong KP. Anti-nucleair antibody (ANA) positivity caused by paraneoplastic antibodies due to abundant p53 expression in early hepatic carcinoma. Neth J Med. 2003; 61:300–303.14. Jaskiewicz K. Chasen MR, Robson SC. Differential expression of extracellular matrix proteins and integrins in hepatocellular carcinoma and chronic liver disease. Anticancer Res. 1993; 13:2229–2237.15. Moore S, Knudsen B, True LD, et al. Loss of stearoyl-CoA desaturase expression is a frequent event in prostate carcinoma. Int J Cancer. 2005; 114:563–571.
Article16. Falvella FS, Pascale RM, Gariboldi M, et al. Stearoyl-CoA desaturase 1 (SCD1) gene overexpression is associated with genetic predisposition to hepatocarcinogenesis in mice and rats. Carcinogenesis. 2002; 23:1933–1936.
Article17. Jiang J, Nilsson-Ehle P, Xu N. Influence of liver cancer on lipid and lipoprotein metabolism. Lipids Health Dis. 2006; 5:4.
Article18. Yahagi N, Shimano H, Hasegawa K, et al. Co-ordinate acti-vation of lipogenic enzymes in hepatocellular carcinoma. Eur J Cancer. 2005; 41:1316–1322.
Article19. Huang J, Zhang X, Zhang M, et al. Upregulation of DLK1 as an imprinted gene could contribute to human hepatocellular carcinoma. Carcinogenesis. 2007; 28:1094–1103.
Article20. Lencioni RA, Allgaier HP, Cioni D, et al. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology. 2003; 228:235.
Article21. Liao C, Zhao M, Song H, Uchida K, Yokoyama KK, Li T. Identification of the gene for a novel liver-related putative tumor suppressor at a high-frequency loss of heterozygosity region of chromosome 8p23 in human hepatocellular carcinoma. Hepatology. 2000; 32:721–727.
Article22. Shirahashi H, Sakaida I, Terai S, Hironaka K, Kusano N, Okita K. Ubiquitin is a possible new predictive marker for the recurrence of human hepatocellular carcinoma. Liver. 2002; 22:413–418.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Analysis of Human V-erbA Related EAR-3 Gene Expression between Transitional Cell Carcinoma and Normal Tissue in Bladder Cancer
- Integrative Analysis of Microarray Data to Reveal Regulation Patterns in the Pathogenesis of Hepatocellular Carcinoma
- Identification of Up-Regulated Genes in Malignant Glioma with Subtraction Hybridization: Preliminary Screening Studies
- Subclassification and Detection of New Markers for the Discrimination of Primary Liver Tumors by Gene Expression Analysis Using Oligonucleotide Arrays
- Analysis of MDR1(multidrug resistance) gene expression in bladder tumor and renal cell carcinoma by polymerase chain reaction