Ann Lab Med.
2014 Mar;34(2):118-126. 10.3343/alm.2014.34.2.118.
Development of Enzyme-Linked Immunosorbent Assays Using 2 Truncated ORF2 Proteins for Detection of IgG Antibodies Against Hepatitis E Virus
- Affiliations
-
- 1Health Research Institute, Infectious and Tropical Disease Research Center, Ahvaz Jundishapur University of Medical Scien, Ahvaz, Iran. f.farshadpour@yahoo.com
- 2Department of Medical Virology, School of Medicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
- KMID: 1791904
- DOI: http://doi.org/10.3343/alm.2014.34.2.118
Abstract
- BACKGROUND
Without appropriate culture systems for hepatitis E virus (HEV), sufficient natural viral proteins are difficult to generate for use in serological tests. Therefore, it is important to produce large amounts of HEV recombinant proteins in an economical way. The present study developed ELISAs using 2 truncated forms of the HEV open reading frame (ORF) 2 protein in order to detect anti-HEV IgG in serum samples.
METHODS
Two truncated forms of the ORF2 protein were expressed in Escherichia coli and were purified by Ni2+-chelate-affinity chromatography (Qiagen, Germany). Two ELISAs were developed using these proteins and were compared with DIA.PRO HEV IgG ELISA kit (DIA.PRO. Italy) in 220 serum samples.
RESULTS
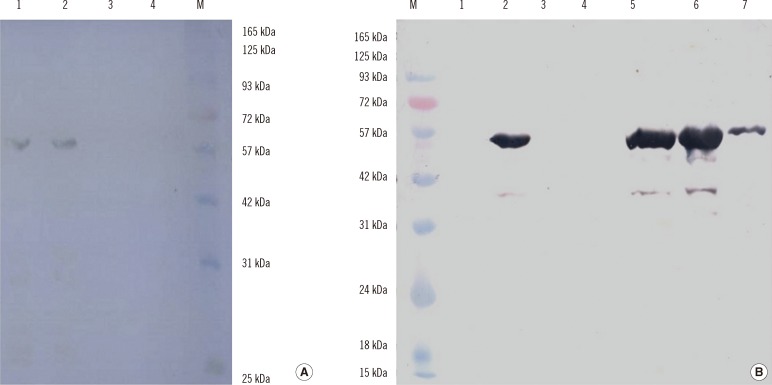
High yields of the target proteins were obtained through codon optimization. The concentration and purity of the proteins were improved with Amicon filters (EMD Millipore, USA). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting analysis of the resultant proteins showed a protein band of approximately 60 kDa corresponding to ORF2.1 (amino acids 112-660) and a protein band of approximately 55 kDa corresponding to ORF2.2 (amino acids 112-607). Positive agreement, negative agreement, and concordance of the 2 in-house ELISAs compared with DIA.PRO HEV IgG ELISA kit were 87%, 99.5%, and 98.1%, respectively (kappa=0.899, P=0.625).
CONCLUSIONS
The newly developed ELISAs are useful for detecting anti-HEV IgG in serum samples and are highly concordant with DIA.PRO HEV IgG ELISA kit.
Keyword
MeSH Terms
-
Amino Acid Sequence
Antibodies/*blood
*Enzyme-Linked Immunosorbent Assay
Escherichia coli/metabolism
Hepatitis E virus/*metabolism
Humans
Immunoglobulin G/*blood
Molecular Sequence Data
Recombinant Proteins/biosynthesis/immunology/isolation & purification
Sequence Alignment
Viral Proteins/chemistry/*immunology/metabolism
Antibodies
Immunoglobulin G
Recombinant Proteins
Viral Proteins
Figure
Reference
-
1. Srivastava R, Aggarwal R, Jameel S, Puri P, Gupta VK, Ramesh VS, et al. Cellular immune responses in acute hepatitis E virus infection to the viral open reading frame 2 protein. Viral Immunol. 2007; 20:56–65. PMID: 17425421.
Article2. Zhu FC, Zhang J, Zhang XF, Zhou C, Wang ZZ, Huang SJ, et al. Efficacy and safety of a recombinant hepatitis E vaccine in healthy adults: a large-scale, randomised, double-blind placebo-controlled, phase 3 trial. Lancet. 2010; 376:895–902. PMID: 20728932.
Article3. Aggarwal R, Shukla R, Jameel S, Agrawal S, Puri P, Gupta VK, et al. T cell epitope mapping of ORF2 and ORF3 proteins of human hepatitis E virus. J Viral Hepat. 2007; 14:283–292. PMID: 17381721.4. Yamashita T, Mori Y, Miyazaki N, Cheng RH, Yoshimura M, Unno H, et al. Biological and immunological characteristics of hepatitis E virus-like particles based on the crystal structure. Proc Natl Acad Sci USA. 2009; 106:12986–12991. PMID: 19620712.
Article5. Uchida T. Cloning and sequencing of cDNA of the hepatitis E virus genome--application to diagnosis. Nihon Rinsho. 1993; 51:352–356. PMID: 8464151.6. Zhou YX, Lee MY, Ng JM, Chye ML, Yip WK, Zee SY, et al. A truncated hepatitis E virus ORF2 protein expressed in tobacco plastids is immunogenic in mice. World J Gastroenterol. 2006; 12:306–312. PMID: 16482635.
Article7. Xing L, Wang JC, Li TC, Yasutomi Y, Lara J, Khudyakov Y, et al. Spatial configuration of hepatitis E virus antigenic domain. J Virol. 2011; 85:1117–1124. PMID: 21068233.
Article8. Zhang J, Liu CB, Li RC, Li YM, Zheng YJ, Li YP, et al. Randomized-controlled phase II clinical trial of a bacterially expressed recombinant hepatitis E vaccine. Vaccine. 2009; 27:1869–1874. PMID: 19168109.
Article9. Emerson SU, Purcell RH. Recombinant vaccines for hepatitis E. Trends Mol Med. 2001; 7:462–466. PMID: 11597521.
Article10. Jiménez de Oya N, Galindo I, Gironés O, Duizer E, Escribano JM, Saiz JC. Serological immunoassay for detection of hepatitis E virus on the basis of genotype 3 open reading frame 2 recombinant proteins produced in Trichoplusia ni larvae. J Clin Microbiol. 2009; 47:3276–3282. PMID: 19656986.11. Sehgal D, Malik PS, Jameel S. Purification and diagnostic utility of a recombinant hepatitis E virus capsid protein expressed in insect larvae. Protein Expr Purif. 2003; 27:27–34. PMID: 12509981.
Article12. Naik S, Aggarwal R, Naik SR, Dwivedi S, Talwar S, Tyagi SK, et al. Evidence for activation of cellular immune responses in patients with acute hepatitis E. Indian J Gastroenterol. 2002; 21:149–152. PMID: 12385543.13. Zhou YH, Purcell RH, Emerson SU. A truncated ORF2 protein contains the most immunogenic site on ORF2: antibody responses to non-vaccine sequences following challenge of vaccinated and non-vaccinated macaques with hepatitis E virus. Vaccine. 2005; 23:3157–3165. PMID: 15837215.
Article14. Emerson SU, Clemente-Casares P, Moiduddin N, Arankalle VA, Torian U, Purcell RH. Putative neutralization epitopes and broad cross-genotype neutralization of Hepatitis E virus confirmed by a quantitative cell-culture assay. J Gen Virol. 2006; 87:697–704. PMID: 16476993.
Article15. Tsarev SA, Tsareva TS, Emerson SU, Govindarajan S, Shapiro M, Gerin JL, et al. Successful passive and active immunization of cynomolgus monkeys against hepatitis E. Proc Natl Acad Sci U S A. 1994; 91:10198–10202. PMID: 7937861.
Article16. Zhang M, Emerson SU, Nguyen H, Engle RE, Govindarajan S, Gerin JL, et al. Immunogenicity and protective efficacy of a vaccine prepared from 53 kDa truncated hepatitis E virus capsid protein expressed in insect cells. Vaccine. 2001; 20:853–857. PMID: 11738749.
Article17. Saiz JC, Gonzalez MJ, Borca MV, Sobrino F, Moore DM. Identification of neutralizing antigenic sites on VP1 and VP2 of type A5 foot-and-mouth disease virus, defined by neutralization-resistant variants. J Virol. 1991; 65:2518–2524. PMID: 1707983.
Article18. Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007; 24:1596–1599. PMID: 17488738.
Article19. Yang EC, Chi BR, Li X, Liu Y, Gao P, Jia P, et al. Expression and characterization of a recombinant truncated capsid protein of hepatitis E virus in Pichia pastoris. Chem Res Chin Univ. 2010; 26:235–239.20. Gholipour A, Moosavian M, Galehdari H, Makvandi M, Memari HR, Alvandi A. Cloning and periplasmic expression of peptidoglycan-associated lipoprotein (PAL) protein of Legionella pneumophila in Escherichia coli. Jundishapur J Microbiol. 2010; 3:1–9.21. Hao BC, Lan X, Xing XY, Xiang HT, Wen FQ, Hu YY, et al. Cloning and prokaryotic expression of cDNAs from hepatitis E virus structural gene of the SW189 strain. Thai J Vet Med. 2012; 42:179–184.22. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976; 72:248–254. PMID: 942051.
Article23. Jacobs D, Vercammen M, Saman E. Evaluation of recombinant dense granule antigen 7 (GRA7) of Toxoplasma gondii for detection of immunoglobulin G antibodies and analysis of a major antigenic domain. Clin Diagn Lab Immunol. 1999; 6:24–29. PMID: 9874659.24. Liu XS, Wang Y, Zhang Y, Fang T, Pan L, Lü J, et al. Cloning, codon-optimized expression and homology modeling of structural protein VP1 from foot and mouth disease virus. Afr J Microbiol Res. 2011; 5:486–495.25. Wang X, Li X, Zhang Z, Shen X, Zhong F. Codon optimization enhances secretory expression of Pseudomonas aeruginosa exotoxin A in E. coli. Protein Expr Purif. 2010; 72:101–106. PMID: 20172029.
Article26. Garmory HS, Brown KA, Titball RW. DNA vaccines: improving expression of antigens. Genet Vaccines Ther. 2003; 1:2. PMID: 14606963.27. Ramakrishna L, Anand KK, Mohankumar KM, Ranga U. Codon optimization of the tat antigen of human immunodeficiency virus type 1 generates strong immune responses in mice following genetic immunization. J Virol. 2004; 78:9174–9189. PMID: 15308713.
Article28. Ruan B, Zumbika E, Wang SY, Chen Y, Ma YL, Chen YG, et al. Cloning and expression of cDNAs from hepatitis E virus structural gene. Hepatobiliary Pancreat Dis Int. 2003; 2:387–390. PMID: 14599945.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Detection of Serum Antibodies to Hepatitis E Virus Based on HEV Genotype 3 ORF2 Capsid Protein Expressed in Nicotiana benthamiana
- Development of a novel enzyme-linked immunosorbent assay to detect anti-IgG against swine hepatitis E virus
- Detection of IgG and IgM Antibodies to Purified Keratinolytic Proteinase in Sera from Patients with Dermatophytosis by Enzyme-Linked Immunosorbent Assay
- Purification of Protein Expressed from Three Different Regions of Norovirus (NoV)
- Evaluation of a Novel Array-Based Toxoplasma, Rubella, Cytomegalovirus, and Herpes Simplex Virus IgG Enzyme Linked Immunosorbent Assay and Its Comparison with Virion/Serion Enzyme Linked Immunosorbent Assays