J Vet Sci.
2013 Dec;14(4):467-472. 10.4142/jvs.2013.14.4.467.
Development of a novel enzyme-linked immunosorbent assay to detect anti-IgG against swine hepatitis E virus
- Affiliations
-
- 1Department of Infectious Diseases, College of Veterinary Medicine, Seoul National University, Seoul 151-742, Korea. yoohs@snu.ac.kr
- KMID: 1712314
- DOI: http://doi.org/10.4142/jvs.2013.14.4.467
Abstract
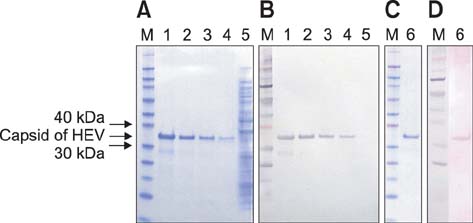
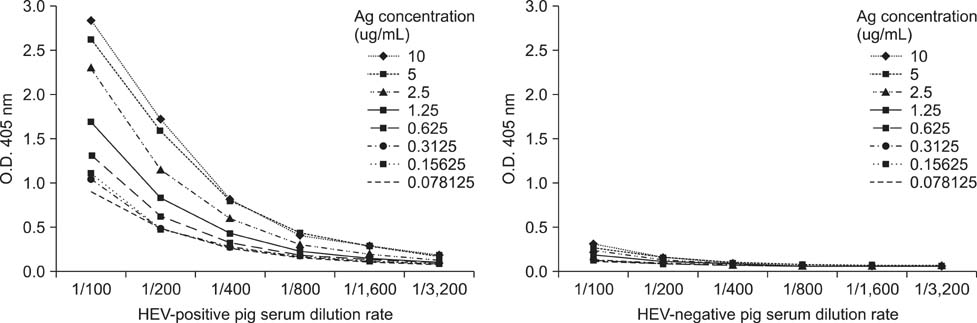
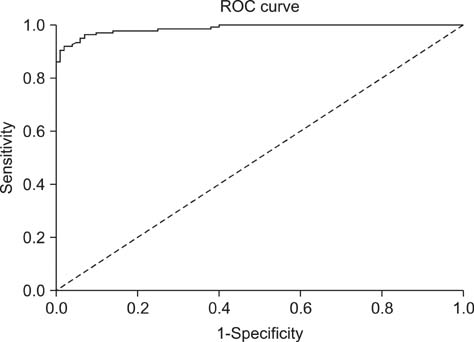
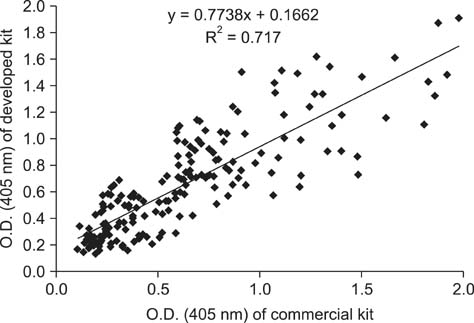
- Swine hepatitis E virus (HEV) is widespread throughout pigs in both developing and industrialized countries. This virus is an important zoonotic agent and a public concern worldwide. Infected pigs are asymptomatic, so diagnosing swine HEV relies on detection of the virus or antibodies against the virus. However, several obstacles need to be overcome for effective and practical serological diagnosis. In this study, we developed an enzyme-linked immunosorbent assay (ELISA) that used a purified recombinant capsid protein of swine HEV. The potential clinical use of this assay was evaluated by comparing it with a commercial kit (Genelabs Technologies, Diagnostics, Singapore). Results of the ELISA were highly correlated with those of the commercial kit with a sensitivity of 97% and specificity of 95%. ROC (receiving operator characteristic) analysis of the ELISA data produced a value of 0.987 (95% CI, 0.977~0.998, p < 0.01). The cut-off value for the ELISA was also determined using negative pig sera. In summary, the HEV-specific ELISA developed in the present study appears to be both practical and economical.
Keyword
MeSH Terms
-
Animals
Antibodies, Anti-Idiotypic/*analysis/blood/genetics
Capsid Proteins/*genetics/metabolism
Enzyme-Linked Immunosorbent Assay/*methods/veterinary
Hepatitis E/diagnosis/immunology/*veterinary/virology
Hepatitis E virus/genetics/*isolation & purification/metabolism
Immunoglobulin G/blood/genetics
ROC Curve
Recombinant Proteins/genetics/metabolism
Swine
Swine Diseases/*diagnosis/immunology/virology
Antibodies, Anti-Idiotypic
Capsid Proteins
Immunoglobulin G
Recombinant Proteins
Figure
Reference
-
1. Aggarwal R. Diagnosis of hepatitis E. Nat Rev Gastroenterol Hepatol. 2013; 10:24–33.
Article2. Ahn JM, Kang SG, Lee DY, Shin SJ, Yoo HS. Identification of novel human hepatitis E virus (HEV) isolates and determination of the seroprevalence of HEV in Korea. J Clin Microbiol. 2005; 43:3042–3048.
Article3. Arankalle VA, Tsarev SA, Chadha MS, Alling DW, Emerson SU, Banerjee K, Purcell RH. Age-specific prevalence of antibodies to hepatitis A and E viruses in Pune, India, 1982 and 1992. J Infect Dis. 1995; 171:447–450.
Article4. Choi IS, Kwon HJ, Shin NR, Yoo HS. Identification of swine hepatitis E virus (HEV) and prevalence of anti-HEV antibodies in swine and human populations in Korea. J Clin Microbiol. 2003; 41:3602–3608.
Article5. Clemente-Casares P, Pina S, Buti M, Jardi R, Martín M, Bofill-Mas S, Girones R. Hepatitis E virus epidemiology in industrialized countries. Emerg Infect Dis. 2003; 9:448–454.
Article6. Crowther JR. The ELISA guidebook. Methods Mol Biol. 2000; 149:III–IIV. 1–413.7. Daniel HDJ, Warier A, Abraham P, Sridharan G. Age-wise exposure rates to hepatitis E virus in a southern Indian patient population without liver disease. Am J Trop Med Hyg. 2004; 71:675–678.
Article8. Feagins AR, Opriessnig T, Guenette DK, Halbur PG, Meng XJ. Detection and characterization of infectious Hepatitis E virus from commercial pig livers sold in local grocery stores in the USA. J Gen Virol. 2007; 88:912–917.
Article9. Fosgate GT, Scott HM, Jordan ER. Development of a method for Bayesian nonparametric ROC analysis with application to an ELISA for John's disease in dairy cattle. Prev Vet Med. 2007; 81:178–193.
Article10. Frey A, Di Canzio J, Zurakowski D. A statistically defined endpoint titer determination method for immunoassays. J Immunol Methods. 1998; 221:35–41.
Article11. Graff J, Zhou YH, Torian U, Nguyen H, St. Claire M, Yu C, Purcell RH, Emerson SU. Claire M, Yu C, Purcell RH, Emerson SU. Mutations within potential glycosylation sites in the capsid protein of hepatitis E virus prevent the formation of infectious virus particles. J Virol. 2008; 82:1185–1194.
Article12. Greiner M, Sohr D, Göbel P. A modified ROC analysis for the selection of cut-off values and the definition of intermediate results of serodiagnostic tests. J Immunol Methods. 1995; 185:123–132.
Article13. Halbur PG, Kasorndorkbua C, Gilbert C, Guenette D, Potters MB, Purcell RH, Emerson SU, Toth TE, Meng XJ. Comparative pathogenesis of infection of pigs with hepatitis E viruses recovered from a pig and a human. J Clin Microbiol. 2001; 39:918–923.
Article14. Herremans M, Bakker J, Duizer E, Vennema H, Koopmans MPG. Use of serological assays for diagnosis of hepatitis E virus genotype 1 and 3 infections in a setting of low endemicity. Clin Vaccine Immunol. 2007; 14:562–568.
Article15. Lee WJ, Kang ML, Cha SB, Park BK, Choi IS, Yoo HS. Analysis of the helicase gene of Korean swine hepatitis E virus isolates and trends in viral infection. Arch Virol. 2009; 154:1361–1364.
Article16. Lee WJ, Yoo HS. Hepatitis E: Morphology, Pathogenesis and Prevention. In : Berhardt LV, editor. Advances in Medicine and Biology. New York: NOVA Science Publishers;2012. Vol. 39:p. 149–165.17. Li SW, Zhang J, He ZQ, Gu Y, Liu RS, Lin J, Chen YX, Ng MH, Xia NS. Mutational analysis of essential interactions involved in the assembly of hepatitis E virus capsid. J Biol Chem. 2005; 280:3400–3406.
Article18. Meng XJ. Hepatitis E virus: animal reservoirs and zoonotic risk. Vet Microbiol. 2010; 140:256–265.
Article19. Meng XJ. Swine hepatitis E virus: cross-species infection and risk in xenotransplantation. Curr Top Microbiol Immunol. 2003; 278:185–216.20. Meng XJ, Purcell RH, Halbur PG, Lehman JR, Webb DM, Tsareva TS, Haynes JS, Thacker BJ, Emerson SU. A novel virus in swine is closely related to the human hepatitis E virus. Proc Natl Acad Sci U S A. 1997; 94:9860–9865.
Article21. Myint KS, Endy TP, Gibbons RV, Laras K, Mammen MP Jr, Sedyaningsih ER, Seriwatana J, Glass JS, Narupiti S, Corwin AL. Evaluation of diagnostic assays for hepatitis E virus in outbreak settings. J Clin Microbiol. 2006; 44:1581–1583.
Article22. de Niet A, Zaaijer HL, ten Berge I, Weegink CJ, Reesink HW, Beuers U. Chronic hepatitis E after solid organ transplantation. Neth J Med. 2012; 70:261–266.23. Park HK, Jeong SH, Kim JW, Woo BH, Lee DH, Kim HY, Ahn S. Seroprevalence of anti-hepatitis E virus (HEV) in a Korean population: comparison of two commercial anti-HEV assays. BMC Infect Dis. 2012; 12:142.
Article24. Surjit M, Jameel S, Lal SK. The ORF2 protein of hepatitis E virus binds the 5' region of viral RNA. J Virol. 2004; 78:320–328.
Article25. Worm HC, van der, Brandstätter G. Hepatitis E: an overview. Microbes Infect. 2002; 4:657–666.
Article26. Zhang H, Dai X, Shan X, Meng J. The Leu477 and Leu613 of ORF2-encoded protein are critical in forming neutralization antigenic epitope of hepatitis E virus genotype 4. Cell Mol Immunol. 2008; 5:447–456.27. Zhang JZ, Im SWK, Lau SH, Chau TN, Lai ST, Ng SP, Peiris M, Tse C, Ng TK, Ng MH. Occurrence of hepatitis E virus IgM, low avidity IgG serum antibodies, and viremia in sporadic cases of non-A, -B, and -C acute hepatitis. J Med Virol. 2002; 66:40–48.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Relative etiology role of hepatitis B virus and hepatitis C virus in HBsAg-negative patients with chronic liver disease in Korea: determination of serum HBV DNA using polymerase chain reaction and of srum anti-HCV using ELISA
- Generation, characterization, and application in serodiagnosis of recombinant swine vesicular disease virus-like particles
- Mass Production and Characterization of Anti-HBsAg Human Antibody B7 Fd
- Improvement of indirect enzyme-linked immunosorbent assay for detection of Japanese encephalitis virus antibodies in swine sera
- Detection of IgG and IgM Antibodies to Purified Keratinolytic Proteinase in Sera from Patients with Dermatophytosis by Enzyme-Linked Immunosorbent Assay