J Korean Med Sci.
2014 Jul;29(7):934-940. 10.3346/jkms.2014.29.7.934.
Imaging Mass Spectrometry in Papillary Thyroid Carcinoma for the Identification and Validation of Biomarker Proteins
- Affiliations
-
- 1Department of Pathology, Konkuk University School of Medicine, Seoul, Korea. wskim@kuh.ac.kr
- 2Department of Molecular Biotechnology, WCU Program, Konkuk University, Seoul, Korea.
- 3SMART Institute of Advanced Biomedical Science, Konkuk University, Seoul, Korea.
- 4The Research Institute of Medical Science, Konkuk University School of Medicine, Seoul, Korea.
- 5Department of Surgery, Konkuk University School of Medicine, Seoul, Korea.
- KMID: 1789952
- DOI: http://doi.org/10.3346/jkms.2014.29.7.934
Abstract
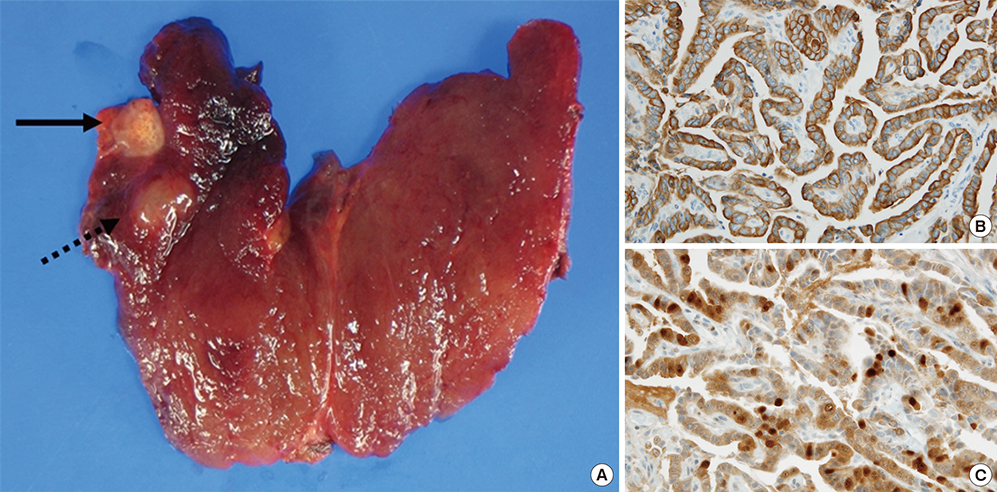
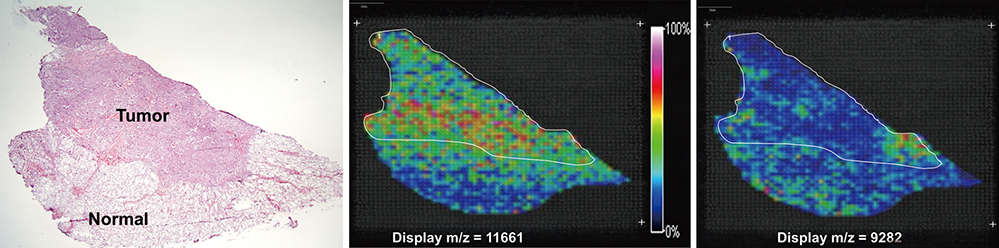
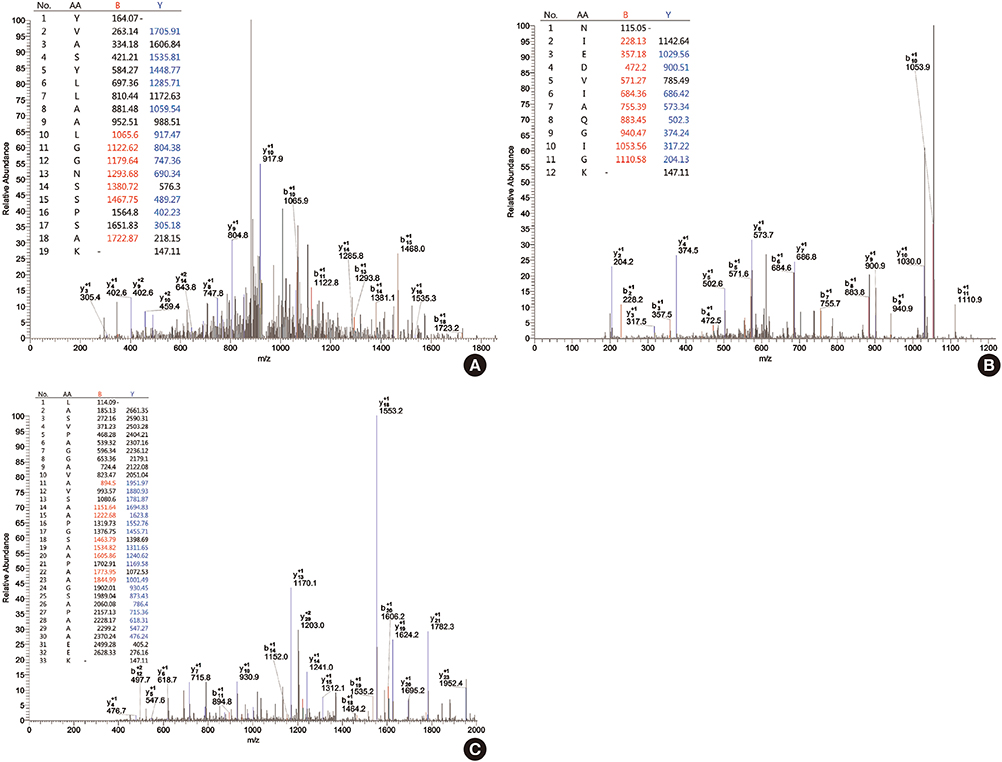
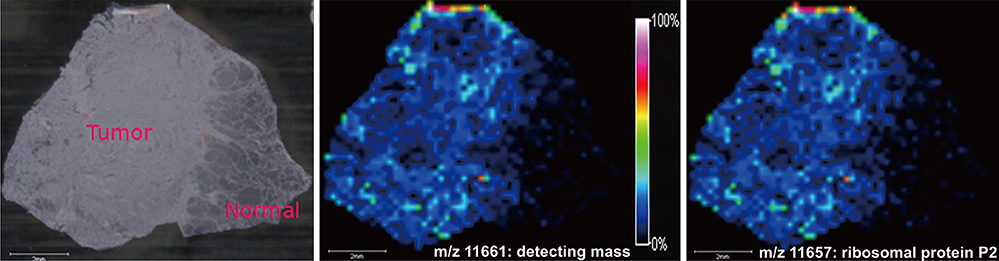
- Direct tissue imaging mass spectrometry (IMS) by matrix-assisted laser desorption ionization and time-of-flight (MALDI-TOF) mass spectrometry has become increasingly important in biology and medicine, because this technology can detect the relative abundance and spatial distribution of interesting proteins in tissues. Five thyroid cancer samples, along with normal tissue, were sliced and transferred onto conductive glass slides. After laser scanning by MALDI-TOF equipped with a smart beam laser, images were created for individual masses and proteins were classified at 200-microm spatial resolution. Based on the spatial distribution, region-specific proteins on a tumor lesion could be identified by protein extraction from tumor tissue and analysis using liquid chromatography with tandem mass spectrometry (LC-MS/MS). Using all the spectral data at each spot, various intensities of a specific peak were detected in the tumor and normal regions of the thyroid. Differences in the molecular weights of expressed proteins between tumor and normal regions were analyzed using unsupervised and supervised clustering. To verify the presence of discovered proteins through IMS, we identified ribosomal protein P2, which is specific for cancer. We have demonstrated the feasibility of IMS as a useful tool for the analysis of tissue sections, and identified the tumor-specific protein ribosomal protein P2.
Keyword
MeSH Terms
-
Aged
Amino Acid Sequence
Biological Markers/*analysis
Carcinoma/*diagnosis/metabolism/pathology
Chromatography, High Pressure Liquid
Cluster Analysis
Female
Humans
Image Processing, Computer-Assisted
Male
Middle Aged
Molecular Sequence Data
Molecular Weight
Phosphoproteins/analysis/metabolism
Proteome/analysis
Proteomics
Reproducibility of Results
Ribosomal Proteins/analysis/metabolism
*Spectrometry, Mass, Matrix-Assisted Laser Desorption-Ionization
*Tandem Mass Spectrometry
Thyroid Gland/metabolism/pathology
Thyroid Neoplasms/*diagnosis/metabolism/pathology
Biological Markers
Phosphoproteins
Proteome
Ribosomal Proteins
Figure
Reference
-
1. Mazzaferri EL. Management of a solitary thyroid nodule. N Engl J Med. 1993; 328:553–559.2. Kondo T, Ezzat S, Asa SL. Pathogenetic mechanisms in thyroid follicular-cell neoplasia. Nat Rev Cancer. 2006; 6:292–306.3. Santarpia L, El-Naggar AK, Cote GJ, Myers JN, Sherman SI. Phosphatidylinositol 3-kinase/akt and ras/raf-mitogen-activated protein kinase pathway mutations in anaplastic thyroid cancer. J Clin Endocrinol Metab. 2008; 93:278–284.4. Xing M. BRAF mutation in papillary thyroid cancer: pathogenic role, molecular bases, and clinical implications. Endocr Rev. 2007; 28:742–762.5. Adeniran AJ, Zhu Z, Gandhi M, Steward DL, Fidler JP, Giordano TJ, Biddinger PW, Nikiforov YE. Correlation between genetic alterations and microscopic features, clinical manifestations, and prognostic characteristics of thyroid papillary carcinomas. Am J Surg Pathol. 2006; 30:216–222.6. Chaurand P, Schwartz SA, Reyzer ML, Caprioli RM. Imaging mass spectrometry: principles and potentials. Toxicol Pathol. 2005; 33:92–101.7. Seeley EH, Caprioli RM. MALDI imaging mass spectrometry of human tissue: method challenges and clinical perspectives. Trends Biotechnol. 2011; 29:136–143.8. Seeley EH, Caprioli RM. Imaging mass spectrometry: towards clinical diagnostics. Proteomics Clin Appl. 2008; 2:1435–1443.9. Ko KH, Kwon CI, Park SH, Han NY, Lee HK, Kim EH, Hahm KB. Application of matrix-assisted laser desorption/ionization time-of-flight imaging mass spectrometry (MALDI-TOF IMS) for premalignant gastrointestinal lesions. Clin Endosc. 2013; 46:611–619.10. Ko KH, Han NY, Kwon CI, Lee HK, Park JM, Kim EH, Hahm KB. Recent advances in molecular imaging of premalignant gastrointestinal lesions and future application for early detection of barrett esophagus. Clin Endosc. 2014; 47:7–14.11. Shimma S, Sugiura Y, Hayasaka T, Hoshikawa Y, Noda T, Setou M. MALDI-based imaging mass spectrometry revealed abnormal distribution of phospholipids in colon cancer liver metastasis. J Chromatogr B Analyt Technol Biomed Life Sci. 2007; 855:98–103.12. Shanta SR, Zhou LH, Park YS, Kim YH, Kim Y, Kim KP. Binary matrix for MALDI imaging mass spectrometry of phospholipids in both ion modes. Anal Chem. 2011; 83:1252–1259.13. Yanagisawa K, Shyr Y, Xu BJ, Massion PP, Larsen PH, White BC, Roberts JR, Edgerton M, Gonzalez A, Nadaf S, et al. Proteomic patterns of tumour subsets in non-small-cell lung cancer. Lancet. 2003; 362:433–439.14. Schwartz SA, Weil RJ, Thompson RC, Shyr Y, Moore JH, Toms SA, Johnson MD, Caprioli RM. Proteomic-based prognosis of brain tumor patients using direct-tissue matrix-assisted laser desorption ionization mass spectrometry. Cancer Res. 2005; 65:7674–7681.15. Cornett DS, Mobley JA, Dias EC, Andersson M, Arteaga CL, Sanders ME, Caprioli RM. A novel histology-directed strategy for MALDI-MS tissue profiling that improves throughput and cellular specificity in human breast cancer. Mol Cell Proteomics. 2006; 5:1975–1983.16. Lemaire R, Menguellet SA, Stauber J, Marchaudon V, Lucot JP, Collinet P, Farine MO, Vinatier D, Day R, Ducoroy P, et al. Specific MALDI imaging and profiling for biomarker hunting and validation: fragment of the 11S proteasome activator complex, Reg alpha fragment, is a new potential ovary cancer biomarker. J Proteome Res. 2007; 6:4127–4134.17. Balluff B, Rauser S, Meding S, Elsner M, Schöne C, Feuchtinger A, Schuhmacher C, Novotny A, Jütting U, Maccarrone G, et al. MALDI imaging identifies prognostic seven-protein signature of novel tissue markers in intestinal-type gastric cancer. Am J Pathol. 2011; 179:2720–2729.18. Meding S, Nitsche U, Balluff B, Elsner M, Rauser S, Schöne C, Nipp M, Maak M, Feith M, Ebert MP, et al. Tumor classification of six common cancer types based on proteomic profiling by MALDI imaging. J Proteome Res. 2012; 11:1996–2003.19. Walch A, Rauser S, Deininger SO, Höfler H. MALDI imaging mass spectrometry for direct tissue analysis: a new frontier for molecular histology. Histochem Cell Biol. 2008; 130:421–434.20. Kang S, Lee A, Park YS, Lee SC, Park SY, Han SY, Kim KP, Kim YH, Yoo CW, Kim HK. Alteration in lipid and protein profiles of ovarian cancer: similarity to breast cancer. Int J Gynecol Cancer. 2011; 21:1566–1572.21. Kang HS, Lee SC, Park YS, Jeon YE, Lee JH, Jung SY, Park IH, Jang SH, Park HM, Yoo CW, et al. Protein and lipid MALDI profiles classify breast cancers according to the intrinsic subtype. BMC Cancer. 2011; 11:465.22. Chaurand P, Sanders ME, Jensen RA, Caprioli RM. Proteomics in diagnostic pathology: profiling and imaging proteins directly in tissue sections. Am J Pathol. 2004; 165:1057–1068.23. Gardner-Thorpe J, Ito H, Ashley SW, Whang EE. Ribosomal protein P2: a potential molecular target for antisense therapy of human malignancies. Anticancer Res. 2003; 23:4549–4560.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Development of Decision Tree Software and Protein Profiling using Surface Enhanced Laser Desorption/Ionization - Time of Flight - Mass Spectrometry (SELDI-TOF-MS) in Papillary Thyroid Cancer
- Concurrent Medullay and Papillary Carcinoma of the Thyroid
- A Case of Ectopic Thyroid Papillary Carcinoma with Incidental Papillary Thyroid Microcarcinoma
- A Case of Mixed Follicular-Papillary Thyroid Carcinoma
- Ultrasonographic imaging of papillary thyroid carcinoma variants