J Korean Med Sci.
2004 Jun;19(3):345-351. 10.3346/jkms.2004.19.3.345.
Loss of Heterozygosity on Chromosomes 3p,8p,9p and 17p in the Progression of Squamous Cell Carcinoma of the Larynx
- Affiliations
-
- 1Department of Otolaryngology-HNS, The Catholic University of Korea, College of Medicine, Seoul, Korea.
- 2Department of Clinical Pathology, The Catholic University of Korea, College of Medicine, Seoul, Korea. lys9908@catholic.ac.kr
- 3Department of Pathology, The Catholic University of Korea, College of Medicine, Seoul, Korea.
- KMID: 1786810
- DOI: http://doi.org/10.3346/jkms.2004.19.3.345
Abstract
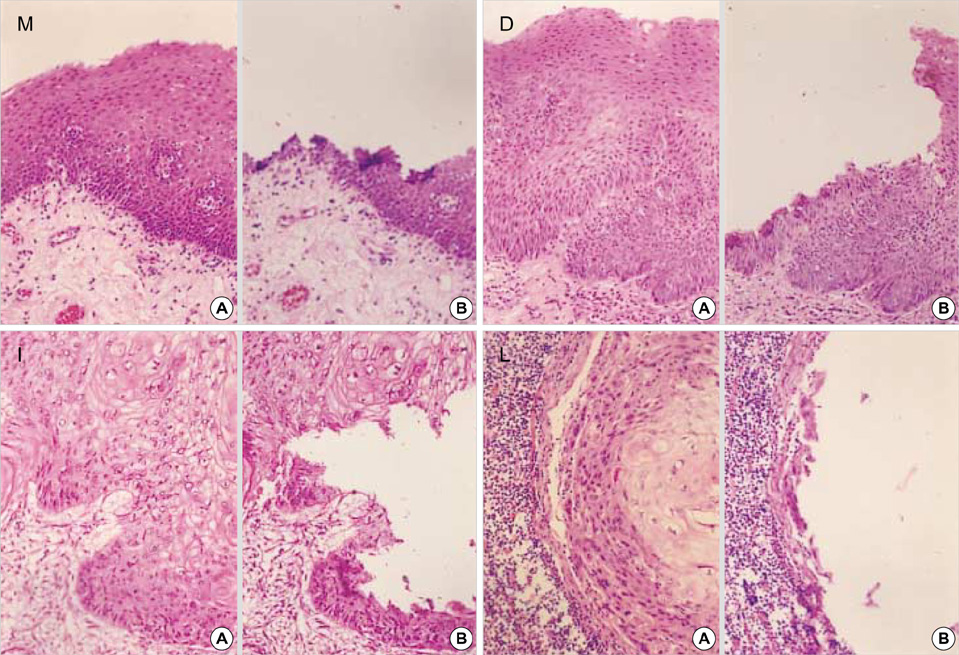
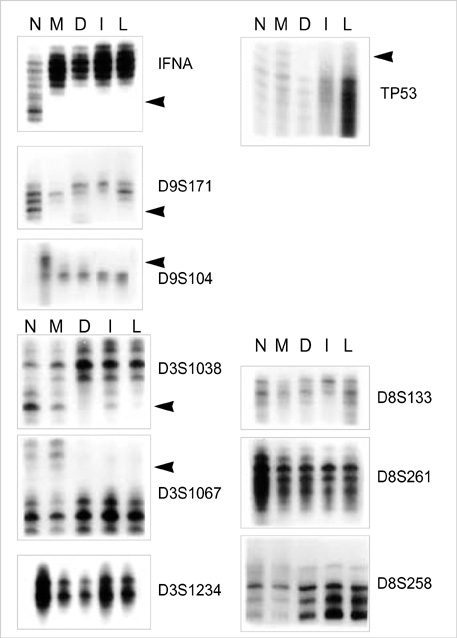
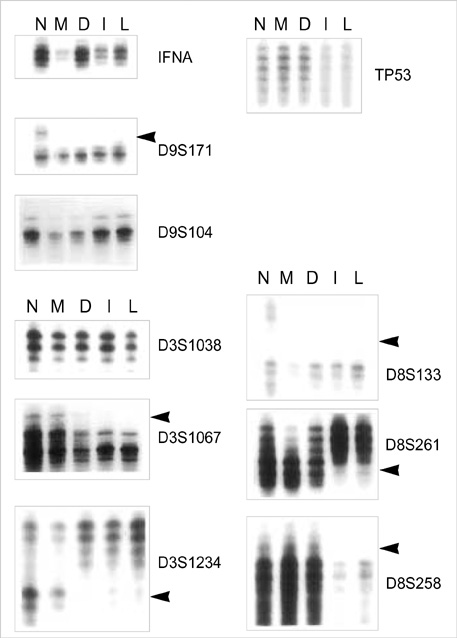
- Previous molecular genetic studies of laryngeal squamous cell carcinoma (SCC)have shown certain chromosomal regions with recurring alterations. But studies of sequential molecular alterations and genetic progression model of laryngeal SCC have not been clearly defined. To identify the chromosomal alterations associated with the carcinogenesis of laryngeal SCC, we analyzed genomic DNA from microdissected squamous metaplasia, squamous dysplasia, invasive SCC, and metastatic carcinoma samples from 22 laryngeal SCC patients for loss of heterozygosity (LOH) at microsatellite loci. Ten microsatellite markers on chromosome 3p, 8p, 9p, and 17p were used. LOH at 9p21 was observed in the all stages including squamous metaplasia, squamous dysplasia, invasive SCC and metastatic carcinoma. LOH at 17p13.1, 3p25 and 3p14.2 was observed from the squamous dysplasia, invasive SCC and metastatic carcinoma. LOH at 8p21.3-p22 was observed mainly from the invasive SCC and metastatic carcinoma. The results suggest that 9p21 in the early event, 17p13.1, 3p25 and 3p14.2 in the intermediate event and 8p21.3- p22 in the late event may be involved in the laryngeal carcinogenesis.
MeSH Terms
-
Carcinoma, Squamous Cell/*genetics/pathology
Chromosome Mapping
*Chromosomes, Human, Pair 17
*Chromosomes, Human, Pair 3
*Chromosomes, Human, Pair 8
*Chromosomes, Human, Pair 9
Disease Progression
Human
Laryngeal Neoplasms/*genetics
Larynx/pathology
*Loss of Heterozygosity
Lymphatic Metastasis
Metaplasia/pathology
Microsatellite Repeats
Neoplasm Metastasis
Figure
Reference
-
1. Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990. 61:759–767.
Article2. Ah-See KW, Cooke TG, Pickford IR, Soutar D, Balmain A. An allelotype of squamous carcinoma of the head and neck using microsatellite markers. Cancer Res. 1994. 54:1617–1621.3. Nawroz H, van der Riet P, Hruban RH, Koch W, Ruppert JM, Sidransky D. Allelotype of head and neck squamous cell carcinoma. Cancer Res. 1994. 54:1152–1155.4. Sidransky D, Mikkelsen T, Schwechheimer K, Rosenblum ML, Cavanee W, Vogelstein B. Clonal expansion of p53 mutant cells is associated with brain tumor progression. Nature. 1992. 355:846–847.5. Simoneau AR, Jones PA. Bladder cancer: the molecular progression to invasive disease. World J Urol. 1994. 12:89–95.
Article6. Califano J, van der Riet P, Westra W, Nawroz H, Clayman G, Piantadosi S, Corio R, Lee D, Greenberg B, Koch W, Sidransky D. Genetic progression model for head and neck cancer: Implications for field cancerization. Cancer Res. 1996. 56:2488–2492.7. Kamb A, Gruis NA, Weaver-Feldhaus J, Liu Q, Harshman K, Tavtigian SV, Stockert E, Day RS 3rd, Johnson BE, Skolnick MH. A cell cycle regulator potentially involved in genesis of many tumor types. Science. 1994. 264:436–440.
Article8. Nobori T, Miura K, Wu DJ, Lois A, Takabayashi K, Carson DA. Deletions of the cyclin-dependent kinase-4 inhibitor gene in multiple human cancers. Nature. 1994. 368:753–756.
Article9. Boyle JO, Hakim J, Koch W, van der Riet P, Hruban RH, Roa RA, Correo R, Eby YJ, Ruppert JM, Sidransky D. The incidence of p53 mutations increases with progression of head and neck cancer. Cancer Res. 1993. 53:4477–4480.10. Soder AI, Hopman AH, Ramaekers FC, Conradt C, Bosch FX. Distinct nonrandom patterns of chromosomal aberrations in the progression of squamous cell carcinomas of the head and neck. Cancer Res. 1995. 55:5030–5037.11. Maestro R, Gasparotto D, Vukosavljevic T, Barzan L, Sulfaro S, Boiocchi M. Three discrete regions of deletion at 3p in head and neck cancers. Cancer Res. 1993. 53:5775–5779.12. Wu CL, Sloan P, Read AP, Harris R, Thakker N. Deletion mapping on the short arm of chromosome 3 in squamous cell carcinoma of the oral cavity. Cancer Res. 1994. 54:6484–6488.13. Latif F, Tory K, Gnarra J, Yao M, Duh FM, Orcutt ML, Stackhouse T, Kuzmin I, Modi W, Geil L, Schmidt L, Zhou F, Li H, Wei MH, Chen F, Glenn G, Choyke P, Walther MU, Weng Y, Duan DSR, Dean M, Glavac D, Richards FM, Crossey PA, Ferguson-Smith MA, Le Pastier D, Chumakov I, Cohen D, Chinault C, Maher ER, Linehan WM, Zbar B, Lerman MI. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science. 1993. 260:1317–1320.
Article14. Ohta M, Inoue H, Cotticelli MG, Kastury K, Baffa R, Palazzo J, Siprashvili Z, Mori M, McCue P, Druck T, Croce CM, Huebner K. The FHIT gene, spanning the chromosome 3p14.2 fragile site and renal carcinoma-associated t(3;8) breakpoint, is abnormal in digestive tract cancers. Cell. 1996. 84:587–597.
Article15. Fujiwara Y, Ohata H, Kuroki T, Koyama K, Tsuchiya E, Monden M, Nakamura Y. Isolation of a candidate tumor suppressor gene on chromosome 8p21.3-p22 that is homologous to an extracellular domain of the PDGF receptor beta gene. Oncogene. 1995. 10:891–895.16. Coleman A, Fountain JW, Nobori T, Olopade OI, Robertson G, Housman DE, Lugo TG. Distinct deletions of chromosome 9p associated with melanoma versus glioma, lung cancer and leukemia. Cancer Res. 1994. 54:344–348.17. Devlin J, Keen AJ, Knowles MA. Homozygous deletion mapping at 9p21 in bladder carcinoma defines a critical region within 2cM of IFNA. Oncogene. 1994. 9:2757–2760.18. Isshiki K, Seng BA, Elder DE, Guerry D, Linnenbach AJ. Chromosome 9 deletion in sporadic and familial melanomas in vivo. Oncogene. 1994. 9:1649–1653.19. Merlo A, Gabrielson E, Mabry M, Vollmer R, Baylin SB, Sidransky D. Homozygous deletion on chromosome 9p and loss of heterozygosity on 9q, 6p, and 6q in primary human small cell lung cancer. Cancer Res. 1994. 54:2322–2326.20. Nees M, Homann N, Discher H, Andl T, Enders C, Herold-Mende C, Schuhmann A, Bosch FX. Expression of mutated p53 occurs in tumor-distant epithelia of head and neck cancer patients: a possible molecular basis for the development of multiple tumors. Cancer Res. 1993. 53:4189–4196.21. Emi M, Fujiwara Y, Nakajima T, Tsuchiya E, Tsuda H, Hirohashi S, Maeda Y, Tsuruta K, Miyaki M, Nakamura Y. Frequent loss of heterozygosity for loci on chromosome 8p in hepatocellular carcinoma, colorectal cancer, and lung cancer. Cancer Res. 1992. 52:5368–5372.22. Chinen K, Isomura M, Izawa K, Fujiwara Y, Ohata H, Iwamasa T, Nakamura Y. Isolation of 45 exon-like fragments from 8p22→p21.3, a region that is commonly deleted in hepatocellular, colorectal, and non-small cell lung carcinomas. Cytogenet Cell Genet. 1996. 75:190–196.23. Scholnick SB, El-Mofty SK, Shaw ME, Sunwoo JB, Haughey BH, Sun PC, Piccirillo JF, Zequeira MR. Clinical correlations with allelotype in supraglottic squamous cancer. Otolaryngol Head Neck Surg. 1998. 118:363–370.
Article24. Ogawara K, Miyakawa A, Shiba M, Uzawa K, Watanabe T, Wang XL, Sato T, Kubosawa H, Kondo Y, Tanzawa H. Allelic loss of chromosome 13q14.3 in human oral cancer: Correlation with lymph node metastasis. Int J Cancer. 1998. 79:312–317.
Article25. Kujawski M, Rydzanicz M, Sarlomo-Rikala M, Szyfter K. Rearrangements involving the 13q chromosome arm committed to the progression of laryngeal squamous cell carcinoma. Cancer Genet Cytogenet. 2002. 137:54–58.
Article26. Brennan JA, Mao L, Hruban R, Boyle JO, Eby YJ, Koch WM, Goodman SN, Sidransky D. Molecular assessment of histopathological staging in squamous-cell carcinoma of the head and neck. N Engl J Med. 1995. 332:429–435.
Article27. Veltman JA, Bot FJ, Huynen FC, Ramaekers FC, Manni JJ, Hopman AH. Chromosome instability as an indicator of malignant progression in laryngeal mucosa. J Clin Oncol. 2000. 18:1644–1651.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- DNA Gains and Losses of Chromosome in Laryngeal Squamous Cell Carcinoma Using Comparative Genomic Hybridization
- Loss of Heterozygosity at VHL, FHIT, and p16 Loci in Nonpapillary Renal Cell Carcinoma
- Loss of Heterozygosity on Chromosome 3p, 9p, 17p in Oral & Oropharyngeal Squamous Cell Carcinoma
- Genetic aberrations on the short arm of chromosome 8 (8p) in tongue carcinomas
- Methylation and Chromosomal Losses in Squamous Cell Carcinoma of the Head and Neck