J Korean Med Sci.
2014 May;29(5):691-698. 10.3346/jkms.2014.29.5.691.
Alkali Therapy Attenuates the Progression of Kidney Injury via Na/H Exchanger Inhibition in 5/6 Nephrectomized Rats
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea. jshan@snu.ac.kr
- 2Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea.
- 3Department of Internal Medicine, Hallym University Hangang Sacred Heart Hospital, Seoul, Korea.
- 4Department of Internal Medicine, Healthcare System Gangnam Center, Seoul National University Hospital, Seoul, Korea.
- 5Epithelial Systems Biology Laboratory, National Heart Lung and Blood Institute, National Institutes of Health, Bethesda, MD, USA.
- KMID: 1786112
- DOI: http://doi.org/10.3346/jkms.2014.29.5.691
Abstract
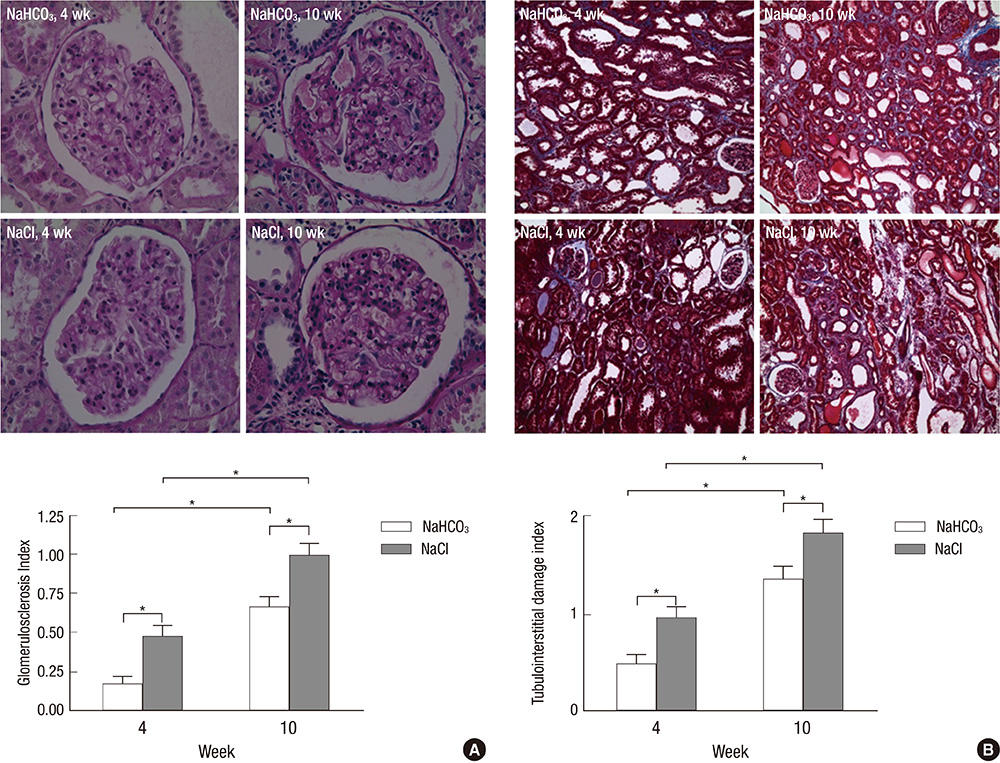
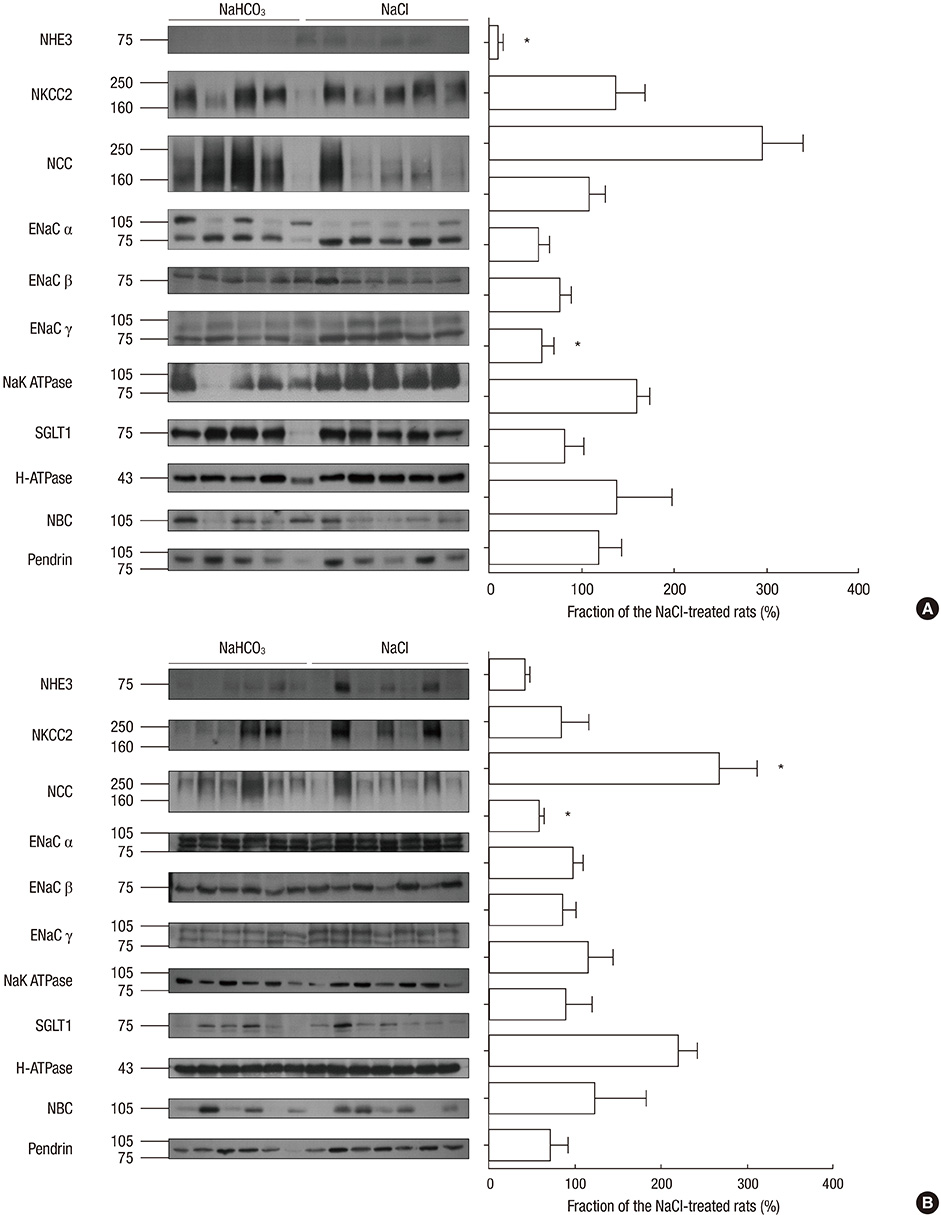
- Metabolic acidosis is a cause of renal disease progression, and alkali therapy ameliorates its progression. However, there are few reports on the role of renal acid-base transporters during alkali therapy. We evaluated the effect of sodium bicarbonate therapy and the role of acid-base transporters on renal disease progression in rats with a remnant kidney. Sprague-Dawley rats consumed dietary sodium bicarbonate (NaHCO3) or sodium chloride (NaCl) with 20% casein after a 5/6 nephrectomy. After being provided with a casein diet, the NaHCO3-treated group had higher levels of serum bicarbonate than the control group. At week 4, the glomerular filtration rate in the NaHCO3 group was higher than that in the NaCl group, and the difference became prominent at week 10. The glomerulosclerosis and tubulointerstitial damage indices in the NaHCO3 group were less severe compared with controls at week 4 and 10. The expression of the Na/H exchanger (NHE) was decreased, and apical reactivity was decreased in the NaHCO3 group, compared with the NaCl group. Endothelin-1 levels in the kidney were also decreased in the NaHCO3 group. Dietary sodium bicarbonate has the effects of ameliorating renal disease progression, which may be related to the altered expression of NHE in the remaining kidney.
MeSH Terms
-
Acidosis/*drug therapy
Alkalies/*therapeutic use
Animals
Caseins/administration & dosage
Disease Progression
Glomerular Filtration Rate/drug effects
Glomerulosclerosis, Focal Segmental/drug therapy
Kidney/injuries
Male
Nephrectomy
Nephritis, Interstitial/drug therapy
Rats
Rats, Sprague-Dawley
Renal Insufficiency/*drug therapy
Sodium Bicarbonate/*therapeutic use
Sodium Chloride/administration & dosage
Sodium-Hydrogen Antiporter/*antagonists & inhibitors
Alkalies
Caseins
Sodium Bicarbonate
Sodium Chloride
Sodium-Hydrogen Antiporter
Figure
Reference
-
1. Frassetto LA, Hsu CY. Metabolic acidosis and progression of chronic kidney disease. J Am Soc Nephrol. 2009; 20:1869–1870.2. De Brito-Ashurst I, Varagunam M, Raftery MJ, Yaqoob MM. Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol. 2009; 20:2075–2084.3. Mahajan A, Simoni J, Sheather SJ, Broglio KR, Rajab MH, Wesson DE. Daily oral sodium bicarbonate preserves glomerular filtration rate by slowing its decline in early hypertensive nephropathy. Kidney Int. 2010; 78:303–309.4. Nath KA, Hostetter MK, Hostetter TH. Pathophysiology of chronic tubulo-interstitial disease in rats. Interactions of dietary acid load, ammonia, and complement component C3. J Clin Invest. 1985; 76:667–675.5. Gadola L, Noboa O, Márquez MN, Rodriguez MJ, Nin N, Boggia J, Ferreiro A, García S, Ortega V, Musto ML, et al. Calcium citrate ameliorates the progression of chronic renal injury. Kidney Int. 2004; 65:1224–1230.6. Phisitkul S, Hacker C, Simoni J, Tran RM, Wesson DE. Dietary protein causes a decline in the glomerular filtration rate of the remnant kidney mediated by metabolic acidosis and endothelin receptors. Kidney Int. 2008; 73:192–199.7. Kim S, Heo NJ, Jung JY, Son MJ, Jang HR, Lee JW, Oh YK, Na KY, Joo KW, Han JS. Changes in the sodium and potassium transporters in the course of chronic renal failure. Nephron Physiol. 2010; 115:p31–p41.8. Okuda S, Tamaki K, Ando T, Nagashima A, Nakayama M, Fukuda K, Higashi H, Fujishima M. Increased expression of Na+/H+ exchanger in the injured renal tissues of focal glomerulosclerosis in rats. Kidney Int. 1994; 46:1635–1643.9. Yamashita J, Ohkita M, Takaoka M, Kaneshiro Y, Matsuo T, Kaneko K, Matsumura Y. Role of Na+/H+ exchanger in the pathogenesis of ischemic acute renal failure in mice. J Cardiovasc Pharmacol. 2007; 49:154–160.10. Amlal H, Chen Q, Greeley T, Pavelic L, Soleimani M. Coordinated down-regulation of NBC-1 and NHE-3 in sodium and bicarbonate loading. Kidney Int. 2001; 60:1824–1836.11. Hropot M, Juretschke HP, Langer KH, Schwark JR. S3226, a novel NHE3 inhibitor, attenuates ischemia-induced acute renal failure in rats. Kidney Int. 2001; 60:2283–2289.12. El Nahas AM, Bassett AH, Cope GH, Le Carpentier JE. Role of growth hormone in the development of experimental renal scarring. Kidney Int. 1991; 40:29–34.13. Oh YK, Joo KW, Lee JW, Jeon US, Lim CS, Han JS, Knepper MA, Na KY. Altered renal sodium transporter expression in an animal model of type 2 diabetes mellitus. J Korean Med Sci. 2007; 22:1034–1041.14. Na KY, Kim GH, Joo KW, Lee JW, Jang HR, Oh YK, Jeon US, Chae SW, Knepper MA, Han JS. Chronic furosemide or hydrochlorothiazide administration increases H+-ATPase B1 subunit abundance in rat kidney. Am J Physiol Renal Physiol. 2007; 292:F1701–F1709.15. Linz WJ, Busch AE. NHE-1 inhibition: from protection during acute ischaemia/reperfusion to prevention/reversal of myocardial remodelling. Naunyn Schmiedebergs Arch Pharmacol. 2003; 368:239–246.16. Cingolani HE, Rebolledo OR, Portiansky EL, Pérez NG, Camilión de Hurtado MC. Regression of hypertensive myocardial fibrosis by Na(+)/H(+) exchange inhibition. Hypertension. 2003; 41:373–377.17. Kim YH, Kwon TH, Frische S, Kim J, Tisher CC, Madsen KM, Nielsen S. Immunocytochemical localization of pendrin in intercalated cell subtypes in rat and mouse kidney. Am J Physiol Renal Physiol. 2002; 283:F744–F754.18. Laghmani K, Borensztein P, Ambühl P, Froissart M, Bichara M, Moe OW, Alpern RJ, Paillard M. Chronic metabolic acidosis enhances NHE-3 protein abundance and transport activity in the rat thick ascending limb by increasing NHE-3 mRNA. J Clin Invest. 1997; 99:24–30.19. Wesson DE, Nathan T, Rose T, Simoni J, Tran RM. Dietary protein induces endothelin-mediated kidney injury through enhanced intrinsic acid production. Kidney Int. 2007; 71:210–217.20. Licht C, Laghmani K, Yanagisawa M, Preisig PA, Alpern RJ. An autocrine role for endothelin-1 in the regulation of proximal tubule NHE3. Kidney Int. 2004; 65:1320–1326.21. Laghmani K, Preisig PA, Alpern RJ. The role of endothelin in proximal tubule proton secretion and the adaptation to a chronic metabolic acidosis. J Nephrol. 2002; 15:S75–S87.22. Eiam-Ong S, Hilden SA, King AJ, Johns CA, Madias NE. Endothelin-1 stimulates the Na+/H+ and Na+/HCO3-transporters in rabbit renal cortex. Kidney Int. 1992; 42:18–24.23. Bobulescu IA, Moe OW. Luminal Na(+)/H (+) exchange in the proximal tubule. Pflugers Arch. 2009; 458:5–21.24. Attmane-Elakeb A, Mount DB, Sibella V, Vernimmen C, Hebert SC, Bichara M. Stimulation by in vivo and in vitro metabolic acidosis of expression of rBSC-1, the Na+-K+(NH4+)-2Cl-cotransporter of the rat medullary thick ascending limb. J Biol Chem. 1998; 273:33681–33691.25. Kim GH, Martin SW, Fernandez-Llama P, Masilamani S, Packer RK, Knepper MA. Long-term regulation of renal Na-dependent cotransporters and ENaC: response to altered acid-base intake. Am J Physiol Renal Physiol. 2000; 279:F459–F467.26. Fukuda Y, Aperia A. Differentiation of Na+-K+ pump in rat proximal tubule is modulated by Na+-H+ exchanger. Am J Physiol. 1988; 255:F552–F557.27. Bhalla V, Hallows KR. Mechanisms of ENaC regulation and clinical implications. J Am Soc Nephrol. 2008; 19:1845–1854.28. Pech V, Pham TD, Hong S, Weinstein AM, Spencer KB, Duke BJ, Walp E, Kim YH, Sutliff RL, Bao HF, et al. Pendrin modulates ENaC function by changing luminal HCO3-. J Am Soc Nephrol. 2010; 21:1928–1941.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Sodium Citrate on Salt Sensitivity and Kidney Injury in Chronic Renal Failure
- Altered Regulation of type 3 Na+/H+ exchanger, type 1 Na+/HCO3- cotransporter, and Na+,K+-ATPase in the Kidney of Rats with Experimental Rhabdomyolysis
- Altered Renal Expression of Acid-base Transporters in Rats with Glycerol-induced Tubular Injury
- Protective Effect of Cariporide(R) against Ischemia/Reperfusion Injury in Rat Kidney
- Alkali therapy for prevention of acute kidney injury in rhabdomyolysis