J Korean Med Sci.
2014 Dec;29(12):1658-1664. 10.3346/jkms.2014.29.12.1658.
Effects of Sodium Citrate on Salt Sensitivity and Kidney Injury in Chronic Renal Failure
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea.
- 2Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea. jshan@snu.ac.kr
- 3Department of Internal Medicine, Hallym University Hangang Sacred Heart Hospital, Seoul, Korea.
- 4Department of Internal Medicine, Healthcare System Gangnam Center, Seoul National University Hospital, Seoul, Korea.
- 5Epithelial Systems Biology Laboratory, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD, USA.
- KMID: 2069955
- DOI: http://doi.org/10.3346/jkms.2014.29.12.1658
Abstract
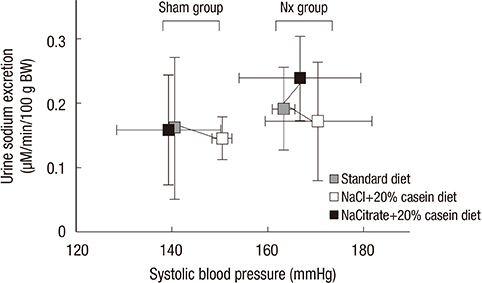
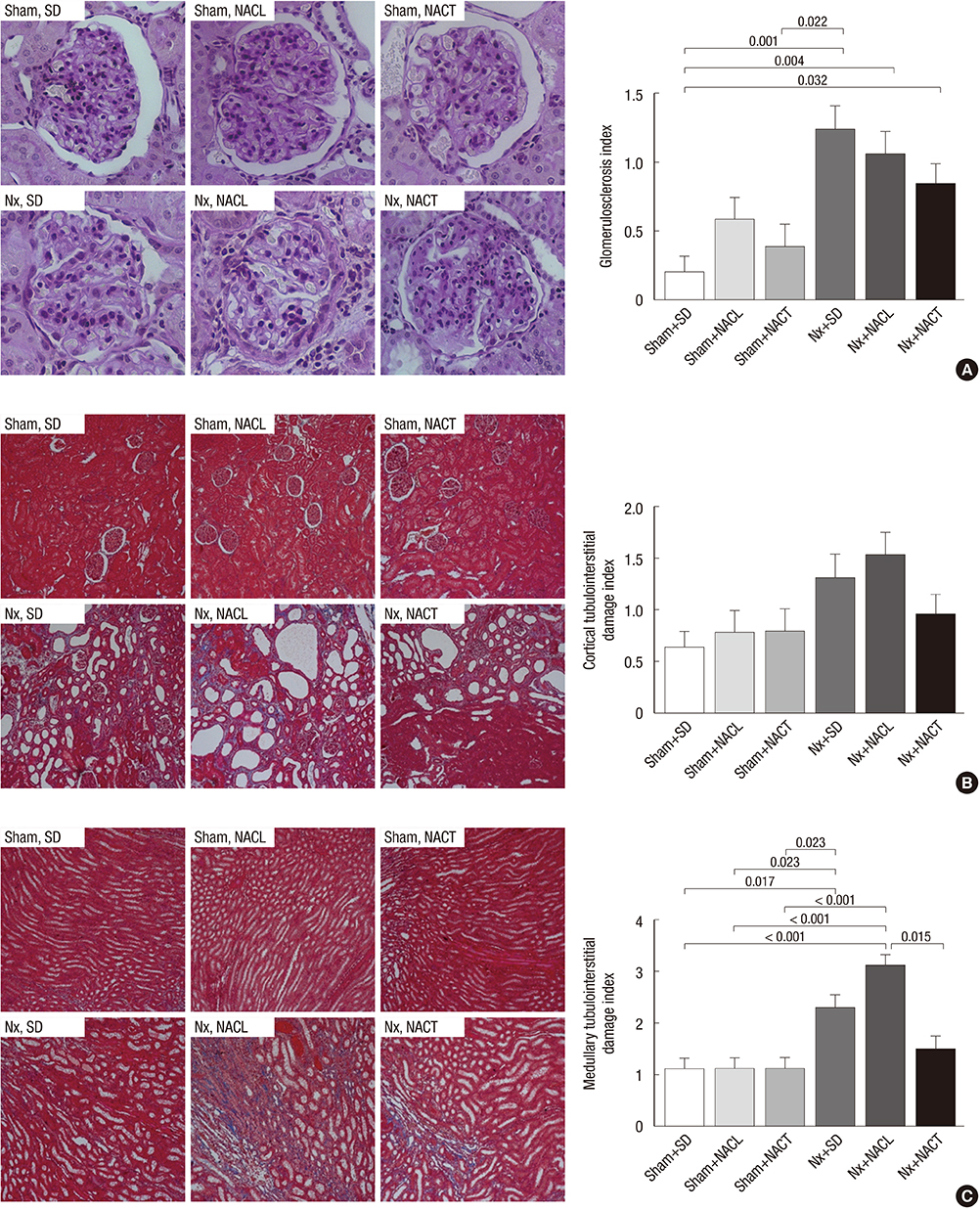
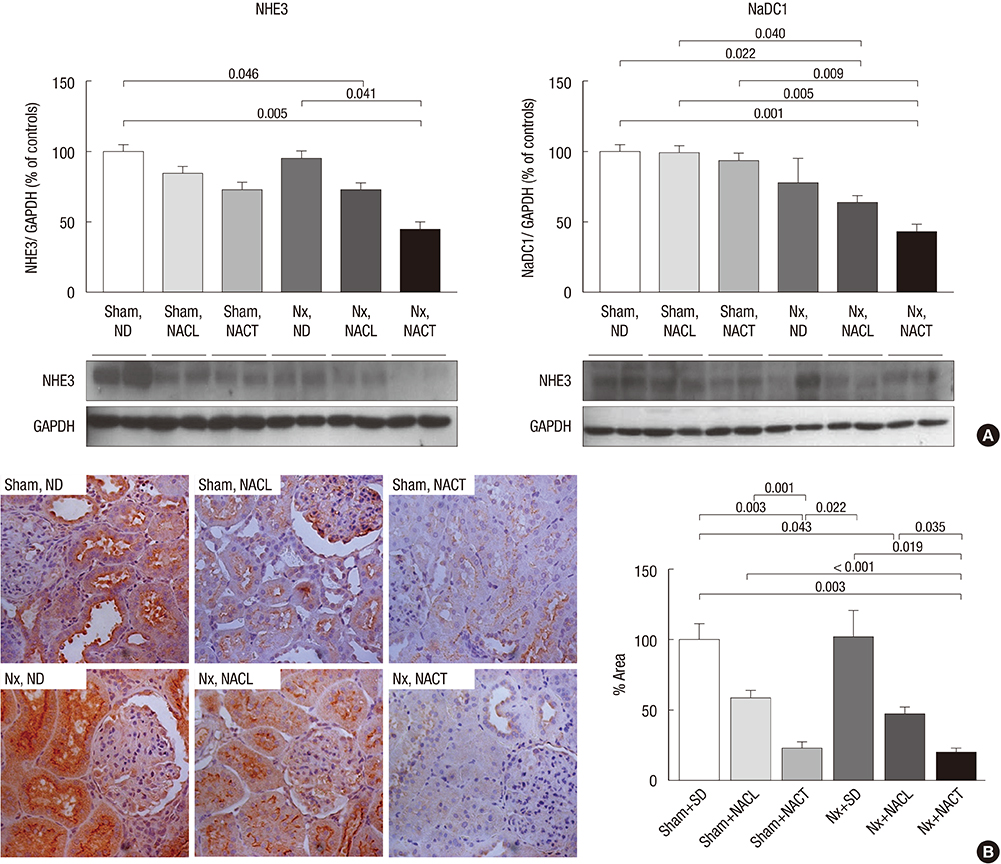
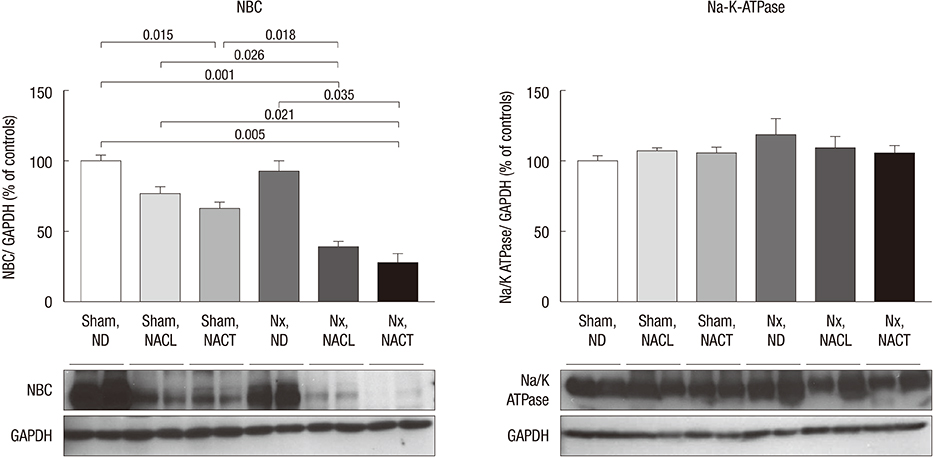
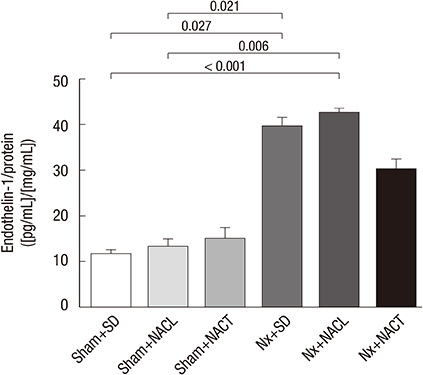
- Metabolic acidosis, which is observed in salt-sensitive hypertension, is also associated with kidney injury. Alkali therapy in chronic renal failure (CRF) may ameliorate the progression of kidney disease; however, few studies have examined the effects of alkali therapy on salt sensitivity and kidney injury in CRF. We randomly administered standard diet (SD), sodium chloride with 20% casein diet (NACL), or sodium citrate with 20% casein diet (NACT) to Sprague-Dawley rats after a CRF or a sham operation. Four weeks after 5/6 nephrectomy, serum bicarbonate levels were higher in the NACT-treated group. On the pressure-natriuresis curve, NACT-treated CRF rats were more salt-resistant than NACL-treated CRF rats. Additionally, the NACT-treated CRF group showed less tubulointerstitial damage than the NACL-treated CRF group. The expression and immunoreactivity of NHE3 in the kidney in the NACT-treated CRF group were lower than those in the NACL-treated CRF group. We observed that dietary NACT as alkali therapy in CRF might improve the altered salt-sensitivity and ameliorate the progression of kidney injury compared to the NACL diet, which may be related to reduced renal NHE3 expression.
Keyword
MeSH Terms
Figure
Reference
-
1. Shah SN, Abramowitz M, Hostetter TH, Melamed ML. Serum bicarbonate levels and the progression of kidney disease: a cohort study. Am J Kidney Dis. 2009; 54:270–277.2. de Brito-Ashurst I, Varagunam M, Raftery MJ, Yaqoob MM. Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol. 2009; 20:2075–2084.3. Mahajan A, Simoni J, Sheather SJ, Broglio KR, Rajab MH, Wesson DE. Daily oral sodium bicarbonate preserves glomerular filtration rate by slowing its decline in early hypertensive nephropathy. Kidney Int. 2010; 78:303–309.4. Raphael KL, Wei G, Baird BC, Greene T, Beddhu S. Higher serum bicarbonate levels within the normal range are associated with better survival and renal outcomes in African Americans. Kidney Int. 2011; 79:356–362.5. Phisitkul S, Khanna A, Simoni J, Broglio K, Sheather S, Rajab MH, Wesson DE. Amelioration of metabolic acidosis in patients with low GFR reduced kidney endothelin production and kidney injury, and better preserved GFR. Kidney Int. 2010; 77:617–623.6. Nath KA, Hostetter MK, Hostetter TH. Pathophysiology of chronic tubulo-interstitial disease in rats. Interactions of dietary acid load, ammonia, and complement component C3. J Clin Invest. 1985; 76:667–675.7. Gadola L, Noboa O, Márquez MN, Rodriguez MJ, Nin N, Boggia J, Ferreiro A, Garcia S, Ortega V, Musto ML, et al. Calcium citrate ameliorates the progression of chronic renal injury. Kidney Int. 2004; 65:1224–1230.8. Phisitkul S, Hacker C, Simoni J, Tran RM, Wesson DE. Dietary protein causes a decline in the glomerular filtration rate of the remnant kidney mediated by metabolic acidosis and endothelin receptors. Kidney Int. 2008; 73:192–199.9. Kim S, Lee J, Heo NJ, Lee JW, Han JS. Alkali therapy attenuates the progression of kidney injury via Na/H exchanger inhibition in 5/6 nephrectomized rats. J Korean Med Sci. 2014; 29:691–698.10. Kim S, Heo NJ, Jung JY, Son MJ, Jang HR, Lee JW, Oh YK, Na KY, Joo KW, Han JS. Changes in the sodium and potassium transporters in the course of chronic renal failure. Nephron Physiol. 2010; 115:p31–p41.11. Morimoto A, Uzu T, Fujii T, Nishimura M, Kuroda S, Nakamura S, Inenaga T, Kimura G. Sodium sensitivity and cardiovascular events in patients with essential hypertension. Lancet. 1997; 350:1734–1737.12. Bigazzi R, Bianchi S, Baldari D, Sgherri G, Baldari G, Campese VM. Microalbuminuria in salt-sensitive patients. A marker for renal and cardiovascular risk factors. Hypertension. 1994; 23:195–199.13. Kimura G, Brenner BM. A method for distinguishing salt-sensitive from non-salt-sensitive forms of human and experimental hypertension. Curr Opin Nephrol Hypertens. 1993; 2:341–349.14. Campese VM. Salt sensitivity in hypertension. Renal and cardiovascular implications. Hypertension. 1994; 23:531–550.15. Sharma AM, Kribben A, Schattenfroh S, Cetto C, Distler A. Salt sensitivity in humans is associated with abnormal acid-base regulation. Hypertension. 1990; 16:407–413.16. Schmouder RL, Weder AB. Platelet sodium-proton exchange is increased in essential hypertension. J Hypertens. 1989; 7:325–330.17. Zhang Y, Magyar CE, Norian JM, Holstein-Rathlou NH, Mircheff AK, McDonough AA. Reversible effects of acute hypertension on proximal tubule sodium transporters. Am J Physiol. 1998; 274:C1090–C1100.18. Griffin KA, Picken M, Bidani AK. Method of renal mass reduction is a critical modulator of subsequent hypertension and glomerular injury. J Am Soc Nephrol. 1994; 4:2023–2031.19. McDonough AA. Mechanisms of proximal tubule sodium transport regulation that link extracellular fluid volume and blood pressure. Am J Physiol Regul Integr Comp Physiol. 2010; 298:R851–R861.20. Wesson DE, Simoni J. Acid retention during kidney failure induces endothelin and aldosterone production which lead to progressive GFR decline, a situation ameliorated by alkali diet. Kidney Int. 2010; 78:1128–1135.21. Ikeda T, Ohta H, Okada M, Kawai N, Nakao R, Siegl PK, Kobayashi T, Maeda S, Miyauchi T, Nishikibe M. Pathophysiological roles of endothelin-1 in Dahl salt-sensitive hypertension. Hypertension. 1999; 34:514–519.22. Amlal H, Chen Q, Greeley T, Pavelic L, Soleimani M. Coordinated down-regulation of NBC-1 and NHE-3 in sodium and bicarbonate loading. Kidney Int. 2001; 60:1824–1836.23. Liu J, Yan Y, Liu L, Xie Z, Malhotra D, Joe B, Shapiro JI. Impairment of Na/K-ATPase signaling in renal proximal tubule contributes to Dahl salt-sensitive hypertension. J Biol Chem. 2011; 286:22806–22813.24. Aruga S, Wehrli S, Kaissling B, Moe OW, Preisig PA, Pajor AM, Alpern RJ. Chronic metabolic acidosis increases NaDC-1 mRNA and protein abundance in rat kidney. Kidney Int. 2000; 58:206–215.25. Simpson DP. Citrate excretion: a window on renal metabolism. Am J Physiol. 1983; 244:F223–F234.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Renal sodium handling and sodium sensitivity
- A Case of Chronic Renal Failure after Exposure to Oral Sodium Phosphate Bowel Purgatives
- Altered Regulation of Renal Aquaporins and Sodium Transporters in Experimental Chronic Renal Failure
- Clinical Experience of Hemodialysis on Three Cases of Renal Failure using Kill Type Artificial Kidney
- Renal Sodium Transporters and Water Channels