Electrolyte Blood Press.
2007 Dec;5(2):55-61. 10.5049/EBP.2007.5.2.55.
Altered Regulation of type 3 Na+/H+ exchanger, type 1 Na+/HCO3- cotransporter, and Na+,K+-ATPase in the Kidney of Rats with Experimental Rhabdomyolysis
- Affiliations
-
- 1Department of Internal Medicine, Chonnam National University Medical School, Gwangju, Korea. skimw@chonnam.ac.kr
- 2Department of Physiology, Chonnam National University Medical School, Gwangju, Korea.
- 3Department of Physiology, Chonbuk National University Medical School, Jeonju, Korea.
- KMID: 2052286
- DOI: http://doi.org/10.5049/EBP.2007.5.2.55
Abstract
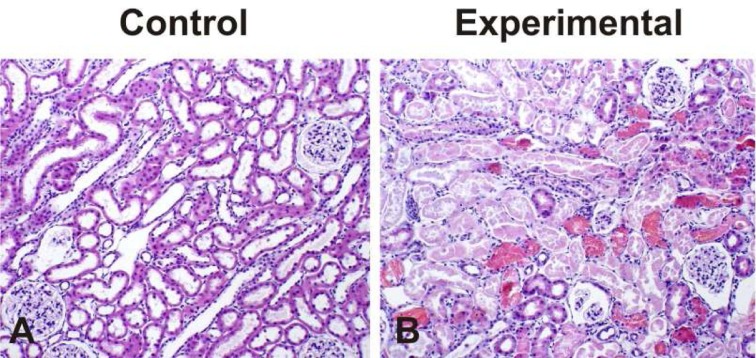
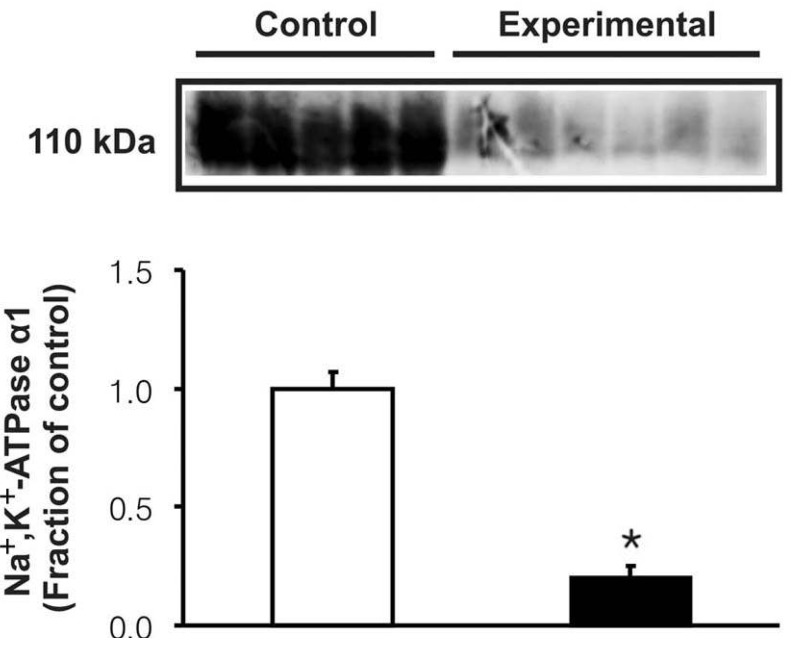
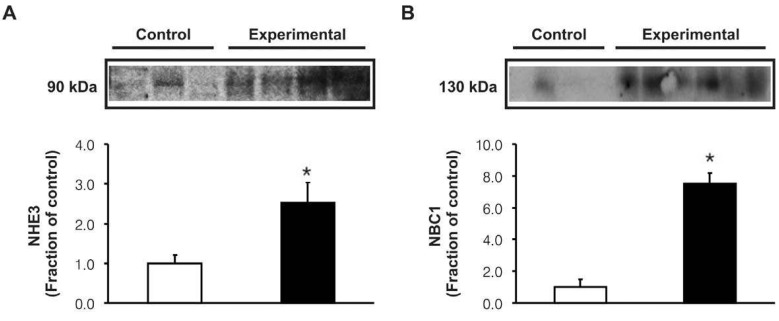
- Metabolic acidosis was shown to correlate with deterioration of renal function in patients with rhabdomyolysis. The present study was aimed to investigate whether the changes of type 3 Na+/H+ exchanger (NHE3), type 1 Na+/HCO3- cotransporter (NBC1), and Na+,K+-ATPase alpha1 subunit may play a role in the pathogenesis of metabolic acidosis in glycerol-induced experimental rhabdomyolysis. Male Sprague-Dawley rats were deprived of fluid intake for 24 hours, and then were injected with 50% glycerol in normal saline (10 mL/kg, intramuscularly). At 24 hours after the glycerol injection, rats were sacrificed by decapitation. Control rats were injected with normal saline. The protein expression of NHE3, NBC1 and Na+,K+-ATPase alpha1 subunit was determined in the cortex of the kidney by immunoblotting and immunohistochemistry. Following the treatment of glycerol, creatinine clearance was significantly decreased, and high anion gap metabolic acidosis developed. In the experimental group, the expression of Na+,K+-ATPase alpha1 subunit was significantly decreased in the cortex of the kidney. On the contrary, the expression of NHE3 and NBC1 was significantly increased. Immunohistochemical analyses confirmed the immunoblotting data. In conclusion, the coordinate up-regulation of NHE3 and NBC1 may play an adaptive role against the metabolic acidosis in glycerol-induced rhabdomyolysis.
MeSH Terms
Figure
Cited by 1 articles
-
Altered Renal Expression of Acid-base Transporters in Rats with Glycerol-induced Tubular Injury
Seong Kwon Ma, Eun Hui Bae, JongUn Lee, Soo Wan Kim
Chonnam Med J. 2010;46(3):163-169. doi: 10.4068/cmj.2010.46.3.163.
Reference
-
1. Vanholder R, Sever MS, Erek E, Lameire N. Rhabdomyolysis. J Am Soc Nephrol. 2000; 11:1553–1561. PMID: 10906171.
Article2. Beetham R. Biochemical investigation of suspected rhabdomyolysis. Ann Clin Biochem. 2000; 37:581–587. PMID: 11026512.
Article3. Paller MS. Hemoglobin- and myoglobin-induced acute renal failure in rats: role of iron in nephrotoxicity. Am J Physiol. 1988; 255:F539–F544. PMID: 3414810.
Article4. Shah SV, Walker PD. Evidence suggesting a role for hydroxyl radical in glycerol-induced acute renal failure. Am J Physiol. 1988; 255:F438–F443. PMID: 2843051.
Article5. Rodrigo R, Bosco C, Herrera P, Rivera G. Amelioration of myoglobinuric renal damage in rats by chronic exposure to flavonol-rich red wine. Nephrol Dial Transplant. 2004; 19:2237–2244. PMID: 15238628.
Article6. Singh D, Chander V, Chopra K. Carvedilol, an antihypertensive drug with antioxidant properties, protects against glycerol-induced acute renal failure. Am J Nephrol. 2003; 23:415–421. PMID: 14573997.
Article7. Nagano T, Mori-Kudo I, Tsuchida A, Kawamura T, Taiji M, Noguchi H. Ameliorative effect of hepatocyte growth factor on glycerol-induced acute renal failure with acute tubular necrosis. Nephron. 2002; 91:730–738. PMID: 12138279.
Article8. Mahnensmith RL, Aronson PS. The plasma membrane sodium-hydrogen exchanger and its role in physiological and pathophysiological processes. Circ Res. 1985; 56:773–788. PMID: 2988813.
Article9. Preisig PA, Ives HE, Cragoe EJ Jr, Alpern RJ, Rector FC Jr. Role of the Na+/H+ antiporter in rat proximal tubule bicarbonate absorption. J Clin Invest. 1987; 80:970–978. PMID: 2888788.10. Soleimani M, Grassi SM, Aronson PS. Stoichiometry of Na+-HCO3- cotransport in basolateral membrane vesicles isolated from rabbit renal cortex. J Clin Invest. 1987; 79:1276–1280. PMID: 3558825.11. Preisig PA, Alpern RJ. Chronic metabolic acidosis causes an adaptation in the apical membrane Na/H antiporter and basolateral membrane Na(HCO3)3 symporter in the rat proximal convoluted tubule. J Clin Invest. 1988; 82:1445–1453. PMID: 2844858.12. Eladari D, Leviel F, Pezy F, Paillard M, Chambrey R. Rat proximal NHE3 adapts to chronic acid-base disorders but not to chronic changes in dietary NaCl intake. Am J Physiol Renal Physiol. 2002; 282:F835–F843. PMID: 11934693.13. Amlal H, Chen Q, Greeley T, Pavelic L, Soleimani M. Coordinated down-regulation of NBC-1 and NHE-3 in sodium and bicarbonate loading. Kidney Int. 2001; 60:1824–1836. PMID: 11703600.
Article14. Muckart DJ, Moodley M, Naidu AG, Reddy AD, Meineke KR. Prediction of acute renal failure following softtissue injury using the venous bicarbonate concentration. J Trauma. 1992; 33:813–817. PMID: 1474620.
Article15. Katz AI, Doucet A, Morel F. Na-K-ATPase activity along the rabbit, rat, and mouse nephron. Am J Physiol. 1979; 237:F114–F120. PMID: 223456.
Article16. Besarab A, Silva P, Epstein FH. Multiple pumps for sodium reabsorption by the perfused kidney. Kidney Int. 1976; 10:147–153. PMID: 135114.
Article17. Kunau RT Jr, Hart JI, Walker KA. Effect of metabolic acidosis on proximal tubular total CO2 absorption. Am J Physiol. 1985; 249:F62–F68. PMID: 3925794.18. Boron WF, Boulpaep EL. Intracellular pH regulation in the renal proximal tubule of the salamander. Basolateral HCO3- transport. J Gen Physiol. 1983; 81:53–94. PMID: 6833997.19. Abuladze N, Lee I, Newman D, Hwang J, Pushkin A, Kurtz I. Axial heterogeneity of sodium-bicarbonate cotransporter expression in the rabbit proximal tubule. Am J Physiol. 1998; 274:F628–F633. PMID: 9530281.20. Schmitt BM, Biemesderfer D, Romero MF, Boulpaep EL, Boron WF. Immunolocalization of the electrogenic Na+-HCO3- cotransporter in mammalian and amphibian kidney. Am J Physiol. 1999; 276:F27–F38. PMID: 9887077.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Altered Renal Expression of Acid-base Transporters in Rats with Glycerol-induced Tubular Injury
- Altered Regulation of NHE3, NBC1 and Nitric Oxide System in the Kidney of Rats with Maleic Acid-nduced Metabolic Acidosis
- Increased Expression of Sodium Transporters in Rats Chronically Inhibited of Nitric Oxide Synthesis
- Altered Regulation of Renal Acid Base Transporters in Response to Ammonium Chloride Loading in Rats
- Endogenous Nitric Oxide Inhibits Na,K-ATPase Activity in the Kidney