J Korean Med Sci.
2014 May;29(5):640-647. 10.3346/jkms.2014.29.5.640.
Preoperative Selective Desensitization of Live Donor Liver Transplant Recipients Considering the Degree of T Lymphocyte Cross-Match Titer, Model for End-Stage Liver Disease Score, and Graft Liver Volume
- Affiliations
-
- 1Department of Surgery, Seoul National University College of Medicine, Seoul, Korea. gsleenj@hanmail.net
- 2Department of Pathology, Seoul National University College of Medicine, Seoul, Korea.
- 3Department of Laboratory Medicine, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 1786103
- DOI: http://doi.org/10.3346/jkms.2014.29.5.640
Abstract
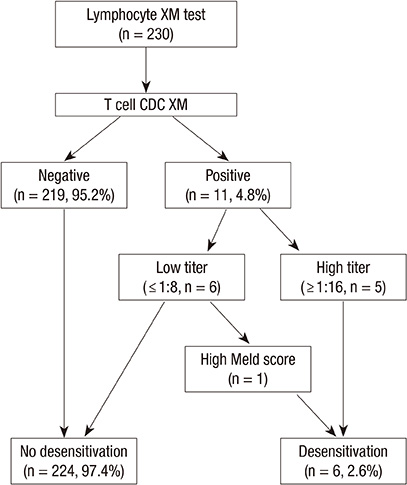
- Several studies have suggested that a positive lymphocyte cross-matching (XM) is associated with low graft survival rates and a high prevalence of acute rejection after adult living donor liver transplantations (ALDLTs) using a small-for-size graft. However, there is still no consensus on preoperative desensitization. We adopted the desensitization protocol from ABO-incompatible LDLT. We performed desensitization for the selected patients according to the degree of T lymphocyte cross-match titer, model for end-stage liver disease (MELD) score, and graft liver volume. We retrospectively evaluated 230 consecutive ALDLT recipients for 5 yr. Eleven recipients (4.8%) showed a positive XM. Among them, five patients with the high titer (> 1:16) by antihuman globulin-augmented method (T-AHG) and one with a low titer but a high MELD score of 36 were selected for desensitization: rituximab injection and plasmapheresis before the transplantation. There were no major side effects of desensitization. Four of the patients showed successful depletion of the T-AHG titer. There was no mortality and hyperacute rejection in lymphocyte XM-positive patients, showing no significant difference in survival outcome between two groups (P=1.000). In conclusion, this desensitization protocol for the selected recipients considering the degree of T lymphocyte cross-match titer, MELD score, and graft liver volume is feasible and safe.
Keyword
MeSH Terms
-
ABO Blood-Group System/immunology
Adult
Antibodies, Monoclonal, Murine-Derived/therapeutic use
Desensitization, Immunologic/*methods
End Stage Liver Disease/surgery
Female
Graft Rejection/immunology
Graft Survival/*immunology
Histocompatibility Testing
Humans
Liver/surgery
*Liver Transplantation
Living Donors
Male
Middle Aged
Plasmapheresis
Preoperative Care
Retrospective Studies
Severity of Illness Index
Survival Rate
T-Lymphocytes/*immunology
*Transplant Recipients
ABO Blood-Group System
Antibodies, Monoclonal, Murine-Derived
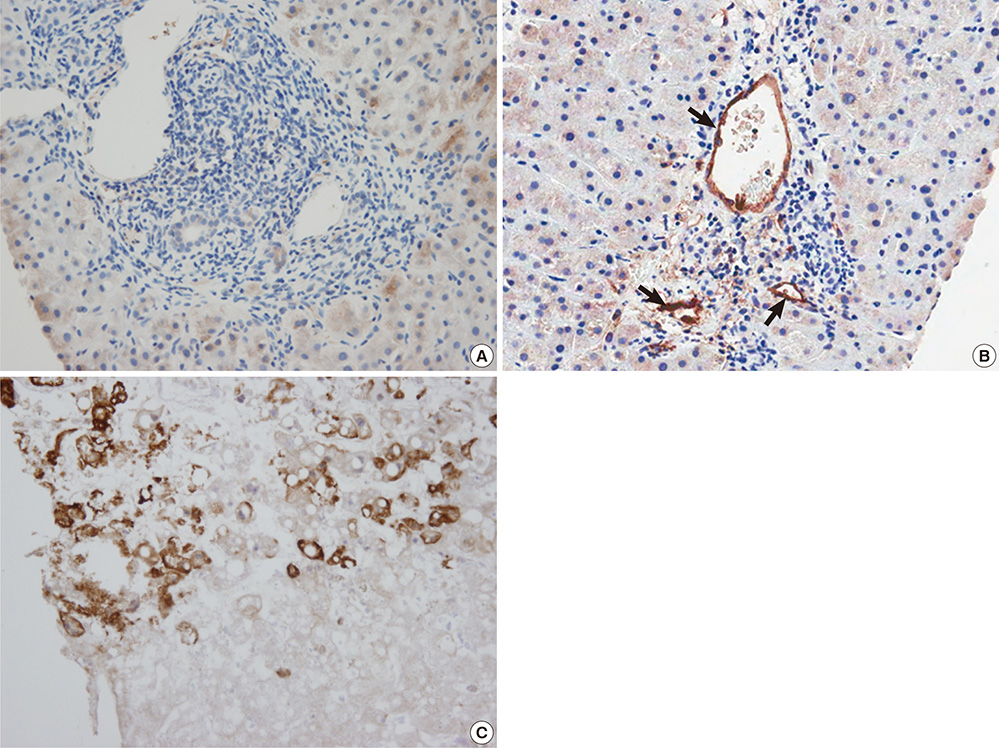
Figure
Reference
-
1. Mulley WR, Kanellis J. Understanding crossmatch testing in organ transplantation: a case-based guide for the general nephrologist. Nephrology (Carlton). 2011; 16:125–133.2. Hori T, Uemoto S, Takada Y, Oike F, Ogura Y, Ogawa K, Miyagawa-Hayashino A, Yurugi K, Nguyen JH, Hori Y, et al. Does a positive lymphocyte cross-match contraindicate living-donor liver transplantation? Surgery. 2010; 147:840–844.3. Yoon HE, Hyoung BJ, Hwang HS, Lee SY, Jeon YJ, Song JC, Oh EJ, Park SC, Choi BS, Moon IS, et al. Successful renal transplantation with desensitization in highly sensitized patients: a single center experience. J Korean Med Sci. 2009; 24:S148–S155.4. Donaldson PT, Alexander GJ, O'Grady J, Neuberger J, Portmann B, Thick M, Davis H, Calne RY, Williams R. Evidence for an immune response to HLA class I antigens in the vanishing-bileduct syndrome after liver transplantation. Lancet. 1987; 1:945–951.5. Goh A, Scalamogna M, De Feo T, Poli F, Terasaki PI. Human leukocyte antigen crossmatch testing is important for liver retransplantation. Liver Transpl. 2010; 16:308–313.6. Suh KS, Kim SB, Chang SH, Kim SH, Minn KW, Park MH, Han KS, Lee KU. Significance of positive cytotoxic cross-match in adult-to-adult living donor liver transplantation using small graft volume. Liver Transpl. 2002; 8:1109–1113.7. Suehiro T, Shimada M, Kishikawa K, Shimura T, Soejima Y, Yoshizumi T, Hashimoto K, Mochida Y, Maehara Y, Kuwano H. Influence of HLA compatibility and lymphocyte cross-matching on acute cellular rejection following living donor adult liver transplantation. Liver Int. 2005; 25:1182–1188.8. Ashihara E, Tsuji H, Sakashita H, Haga H, Yurugi K, Kimura S, Egawa H, Manabe T, Uemoto S, Maekawa T, et al. Antidonor antibody in patients receiving ABO-identical and HLA-mismatched living donor liver transplants: effect on survival. Transplantation. 2007; 83:506–509.9. New York State Committee on Quality improvement in living liver donation. Quality Improvement in Living Liver Donation Health. 2002. accessed on 21 March 2014. Available at http://www.health.ny.gov/professionals/patients/donation/organ/liver.10. Jung KU, Park JB, Kim JM, Moon JI, Jung GO, Chun JM, Choi GS, Kwon CD, Kim SJ, Joh JW, et al. The risk factors of acute cellular rejection in adult living donor liver transplantation: doubting the value of positive lymphocytotoxic cross-match results. J Korean Soc Transplant. 2009; 23:237–243.11. Sugawara Y, Tamura S, Kaneko J, Togashi J, Makuuchi M, Kokudo N. Positive lymphocytotoxic crossmatch does not adversely affect survival in living donor liver transplantation. Dig Surg. 2009; 26:482–486.12. Kamar N, Lavayssière L, Muscari F, Selves J, Guilbeau-Frugier C, Cardeau I, Esposito L, Cointault O, Nogier MB, Peron JM, et al. Early plasmapheresis and rituximab for acute humoral rejection after ABO-compatible liver transplantation. World J Gastroenterol. 2009; 15:3426–3430.13. Muro M, Marin L, Miras M, Moya-Quiles R, Minguela A, Sánchez-Bueno F, Bermejo J, Robles R, Ramírez P, García-Alonso A, et al. Liver recipients harbouring anti-donor preformed lymphocytotoxic antibodies exhibit a poor allograft survival at the first year after transplantation: experience of one centre. Transpl Immunol. 2005; 14:91–97.14. White NB, Greenstein SM, Cantafio AW, Schechner R, Glicklich D, McDonough P, Pullman J, Mohandas K, Boctor F, Uehlinger J, et al. Successful rescue therapy with plasmapheresis and intravenous immunoglobulin for acute humoral renal transplant rejection. Transplantation. 2004; 78:772–774.15. Vaidya S, Cooper TY, Avandsalehi J, Barnes T, Brooks K, Hymel P, Noor M, Sellers R, Thomas A, Stewart D, et al. Improved flow cytometric detection of HLA alloantibodies using pronase: potential implications in renal transplantation. Transplantation. 2001; 71:422–428.16. Jordan SC, Toyoda M, Kahwaji J, Vo AA. Clinical aspects of intravenous immunoglobulin use in solid organ transplant recipients. Am J Transplant. 2011; 11:196–202.17. Shehata N, Palda VA, Meyer RM, Blydt-Hansen TD, Campbell P, Cardella C, Martin S, Nickerson P, Peltekian K, Ross H, et al. The use of immunoglobulin therapy for patients undergoing solid organ transplantation: an evidence-based practice guideline. Transfus Med Rev. 2010; 24:S7–S27.18. Bellamy CO, Herriot MM, Harrison DJ, Bathgate AJ. C4d immunopositivity is uncommon in ABO-compatible liver allografts, but correlates partially with lymphocytotoxic antibody status. Histopathology. 2007; 50:739–749.19. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004; 240:205–213.20. Yi NJ, Suh KS, Lee HW, Cho EH, Shin WY, Cho JY, Lee KU. An artificial vascular graft is a useful interpositional material for drainage of the right anterior section in living donor liver transplantation. Liver Transpl. 2007; 13:1159–1167.21. Doyle HR, Marino IR, Morelli F, Doria C, Aldrighetti L, McMichael J, Martell J, Gayowski T, Starzl TE. Assessing risk in liver transplantation: special reference to the significance of a positive cytotoxic crossmatch. Ann Surg. 1996; 224:168–177.22. Terasaki PI, Cai J. Humoral theory of transplantation: further evidence. Curr Opin Immunol. 2005; 17:541–545.23. Musat AI, Agni RM, Wai PY, Pirsch JD, Lorentzen DF, Powell A, Leverson GE, Bellingham JM, Fernandez LA, Foley DP, et al. The significance of donor-specific HLA antibodies in rejection and ductopenia development in ABO compatible liver transplantation. Am J Transplant. 2011; 11:500–510.24. Watson R, Kozlowski T, Nickeleit V, Woosley JT, Schmitz JL, Zacks SL, Fair JH, Gerber DA, Andreoni KA. Isolated donor specific alloantibody-mediated rejection after ABO compatible liver transplantation. Am J Transplant. 2006; 6:3022–3029.25. Sugawara Y, Makuuchi M, Kaneko J, Kishi Y, Hata S, Kokudo N. Positive T lymphocytotoxic cross-match in living donor liver transplantation. Liver Transpl. 2003; 9:1062–1066.26. Aoki T, Sugawara Y, Takahashi M, Kawaguchi Y, Kaneko J, Yamashiki N, Tamura S, Hasegawa K, Takahashi K, Kokudo N. Living donor liver transplantation using sensitized lymphocytotoxic crossmatch positive graft. J Gastroenterol. 2012; 47:486–488.27. Egawa H, Teramukai S, Haga H, Tanabe M, Fukushima M, Shimazu M. Present status of ABO-incompatible living donor liver transplantation in Japan. Hepatology. 2008; 47:143–152.28. Yao FY, Saab S, Bass NM, Hirose R, Ly D, Terrault N, Lazar AA, Bacchetti P, Ascher NL, Roberts JP. Prediction of survival after liver retransplantation for late graft failure based on preoperative prognostic scores. Hepatology. 2004; 39:230–238.29. Rhee C, Narsinh K, Venick RS, Molina RA, Nga V, Engelhardt R, Martín MG. Predictors of clinical outcome in children undergoing orthotopic liver transplantation for acute and chronic liver disease. Liver Transpl. 2006; 12:1347–1356.30. Rana A, Hardy MA, Halazun KJ, Woodland DC, Ratner LE, Samstein B, Guarrera JV, Brown RS Jr, Emond JC. Survival outcomes following liver transplantation (SOFT) score: a novel method to predict patient survival following liver transplantation. Am J Transplant. 2008; 8:2537–2546.31. Ben-Haim M, Emre S, Fishbein TM, Sheiner PA, Bodian CA, Kim-Schluger L, Schwartz ME, Miller CM. Critical graft size in adult-to-adult living donor liver transplantation: impact of the recipient's disease. Liver Transpl. 2001; 7:948–953.32. Austin GL, Sasaki AW, Zaman A, Rabkin JM, Olyaei A, Ruimy R, Orloff SL, Ham J, Rosen HR. Comparative analysis of outcome following liver transplantation in US veterans. Am J Transplant. 2004; 4:788–795.33. Lunz J, Ruppert KM, Cajaiba MM, Isse K, Bentlejewski CA, Minervini M, Nalesnik MA, Randhawa P, Rubin E, Sasatomi E, et al. Re-examination of the lymphocytotoxic crossmatch in liver transplantation: can C4d stains help in monitoring? Am J Transplant. 2012; 12:171–182.34. Demetris AJ, Nakamura K, Yagihashi A, Iwaki Y, Takaya S, Hartman GG, Murase N, Bronsther O, Manez R, Fung JJ, et al. A clinicopathological study of human liver allograft recipients harboring preformed IgG lymphocytotoxic antibodies. Hepatology. 1992; 16:671–681.35. Kakizoe S, Yanaga K, Starzl TE, Demetris AJ. Evaluation of protocol before transplantation and after reperfusion biopsies from human orthotopic liver allografts: considerations of preservation and early immunological injury. Hepatology. 1990; 11:932–941.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Mortality scoring systems for liver transplant recipients: before and after model for end-stage liver disease score
- Current Status of Deceased Donor Liver Transplantation for Alcoholic Liver Disease in Korea in MELD Era
- Split liver transplantation for two adult recipients
- Experience of split liver transplantation from deceased marginal donor: eight adult recipients from four deceased donors
- Long-term outcomes of emergency ABO-incompatible living donor liver transplantation using a modified desensitization protocol for highly sensitized patients with acute liver failure: A case report