Ann Hepatobiliary Pancreat Surg.
2021 Nov;25(4):571-574. 10.14701/ahbps.2021.25.4.571.
Long-term outcomes of emergency ABO-incompatible living donor liver transplantation using a modified desensitization protocol for highly sensitized patients with acute liver failure: A case report
- Affiliations
-
- 1Department of Surgery, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
- 2Department of Surgery, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- KMID: 2523066
- DOI: http://doi.org/10.14701/ahbps.2021.25.4.571
Abstract
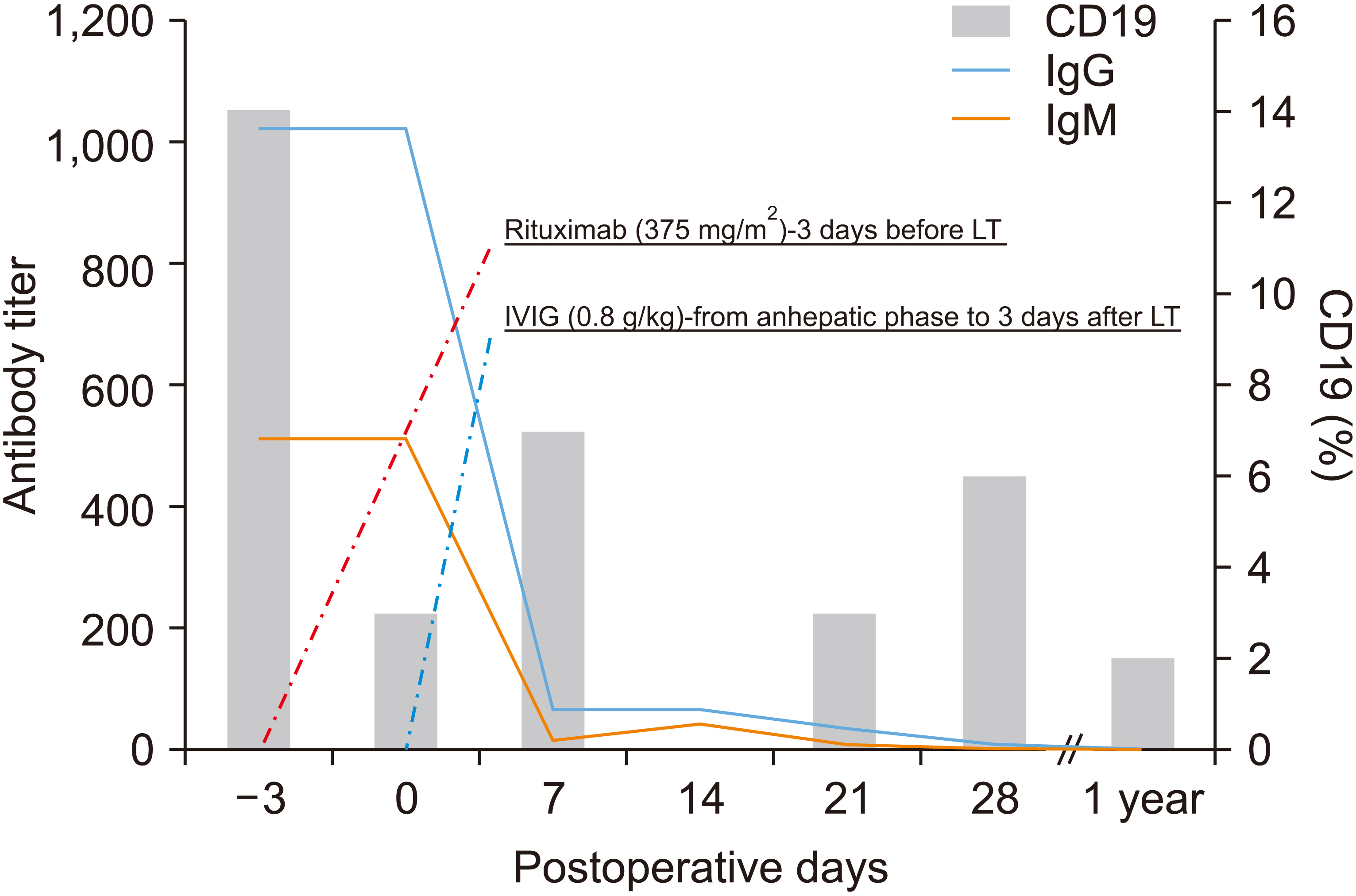
- Although there is no established desensitization protocol for liver transplantation (LT), desensitization usually entails treatment with rituximab, plasmapheresis, splenectomy, and intravenous immunoglobulin (IVIG) infusion together with a local graft. The desensitization protocol is usually initiated 2 to 3 weeks before transplantation. Therefore, patients with acute liver failure warranting urgent LT are usually ineligible for ABO-incompatible (ABOi) LT. For these reasons, several attempts have been made to abridge the desensitization protocol and extend the indication for ABOi living donor LT (LDLT). Here we report a 40-year-old female diagnosed with chronic hepatitis B and acute-on-chronic liver failure (model for end-stage liver disease score, 31). In the absence of a suitable compatible liver donor, emergency ABOi LT was planned using a modified desensitization protocol. The preoperative isoagglutinin (IA) titer was 1 : 1,024 and the preoperative T- and B-cell cross-matches were positive. The patient received a single dose of rituximab (375 mg/ m2 ) and IVIG (0.8 g/kg) was administered from the anhepatic phase until three days after transplantation. Although the patient developed acute cellular rejection in the early stages after LT, she has maintained a stable graft function, even after 5 years. In summary, a modified desensitization protocol consisting of rituximab and IVIG is a feasible strategy for highly sensitized patients with elevated IA titers indicated for urgent LDLT.
Figure
Reference
-
1. Gordon RD, Iwatsuki S, Esquivel CO, Tzakis A, Todo S, Starzl TE. 1986; Liver transplantation across ABO blood groups. Surgery. 100:342–348. DOI: 10.1016/j.transproceed.2003.08.002. PMID: 14529912.2. Egawa H, Teramukai S, Haga H, Tanabe M, Mori A, Ikegami T, et al. 2014; Impact of rituximab desensitization on blood-type-incompatible adult living donor liver transplantation: a Japanese multicenter study. Am J Transplant. 14:102–114. DOI: 10.1111/ajt.12520. PMID: 24279828.
Article3. Usuda M, Fujimori K, Koyamada N, Fukumori T, Sekiguchi S, Kawagishi N, et al. 2005; Successful use of anti-CD20 monoclonal antibody (rituximab) for ABO-incompatible living-related liver transplantation. Transplantation. 79:12–16. DOI: 10.1097/01.TP.0000149337.40911.E4. PMID: 15714163.
Article4. Shen T, Lin BY, Jia JJ, Wang ZY, Wang L, Ling Q, et al. 2014; A modified protocol with rituximab and intravenous immunoglobulin in emergent ABO-incompatible liver transplantation for acute liver failure. Hepatobiliary Pancreat Dis Int. 13:395–401. DOI: 10.1016/S1499-3872(14)60268-X. PMID: 25100124.
Article5. Kim SH, Lee EC, Shim JR, Park SJ. 2018; A simplified protocol using rituximab and immunoglobulin for ABO-incompatible low-titre living donor liver transplantation. Liver Int. 38:932–939. DOI: 10.1111/liv.13614. PMID: 29053910.
Article6. Song GW, Lee SG, Hwang S, Kim KH, Ahn CS, Moon DB, et al. 2016; ABO-incompatible adult living donor liver transplantation under the desensitization protocol with rituximab. Am J Transplant. 16:157–170. DOI: 10.1111/ajt.13444. PMID: 26372830.
Article7. Egawa H, Teramukai S, Haga H, Tanabe M, Fukushima M, Shimazu M. 2008; Present status of ABO-incompatible living donor liver transplantation in Japan. Hepatology. 47:143–152. DOI: 10.1002/hep.21928. PMID: 17929298.
Article8. Haga H, Egawa H, Fujimoto Y, Ueda M, Miyagawa-Hayashino A, Sakurai T, et al. 2006; Acute humoral rejection and C4d immunostaining in ABO blood type-incompatible liver transplantation. Liver Transpl. 12:457–464. DOI: 10.1002/lt.20652. PMID: 16498648.
Article9. Skogsberg U, Breimer ME, Friman S, Mjörnstedt L, Mölne J, Olausson M, et al. 2006; Adult ABO-incompatible liver transplantation, using A and B donors. Xenotransplantation. 13:154–159. DOI: 10.1111/j.1399-3089.2006.00286.x. PMID: 16623811.10. Mendes M, Ferreira AC, Ferreira A, Remédio F, Aires I, Cordeiro A, et al. 2013; ABO-incompatible liver transplantation in acute liver failure: a single Portuguese center study. Transplant Proc. 45:1110–1115. DOI: 10.1016/j.transproceed.2013.02.012. PMID: 23622639.
Article11. Yamamoto H, Uchida K, Kawabata S, Isono K, Miura K, Hayashida S, et al. 2018; Feasibility of monotherapy by rituximab without additional desensitization in ABO-incompatible living-donor liver transplantation. Transplantation. 102:97–104. DOI: 10.1097/TP.0000000000001956. PMID: 28938311.
Article12. Lee EC, Kim SH, Shim JR, Park SJ. 2018; A comparison of desensitization methods: rituximab with/without plasmapheresis in ABO-incompatible living donor liver transplantation. Hepatobiliary Pancreat Dis Int. 17:119–125. DOI: 10.1016/j.hbpd.2018.02.005. PMID: 29576278.
Article13. Lee B, Choi Y, Han HS, Yoon YS, Cho JY, Jeong SH, et al. 2019; ABO-incompatible liver transplantation using only rituximab for patients with low anti-ABO antibody titer. Ann Hepatobiliary Pancreat Surg. 23:211–218. DOI: 10.14701/ahbps.2019.23.3.211. PMID: 31501808. PMCID: PMC6728259.
Article14. John R, Lietz K, Burke E, Ankersmit J, Mancini D, Suciu-Foca N, et al. 1999; Intravenous immunoglobulin reduces anti-HLA alloreactivity and shortens waiting time to cardiac transplantation in highly sensitized left ventricular assist device recipients. Circulation. 100(19 Suppl):II229–II235. DOI: 10.1161/01.CIR.100.suppl_2.II-229. PMID: 10567309.
Article15. Puisset F, White-Koning M, Kamar N, Huart A, Haberer F, Blasco H, et al. 2013; Population pharmacokinetics of rituximab with or without plasmapheresis in kidney patients with antibody-mediated disease. Br J Clin Pharmacol. 76:734–740. DOI: 10.1111/bcp.12098. PMID: 23432476. PMCID: PMC3853532.
Article16. Zarkhin V, Li L, Kambham N, Sigdel T, Salvatierra O, Sarwal MM. 2008; A randomized, prospective trial of rituximab for acute rejection in pediatric renal transplantation. Am J Transplant. 8:2607–2617. DOI: 10.1111/j.1600-6143.2008.02411.x. PMID: 18808404.
Article17. Wang M, Han XH, Zhang L, Yang J, Qian JF, Shi YK, et al. 2008; Bortezomib is synergistic with rituximab and cyclophosphamide in inducing apoptosis of mantle cell lymphoma cells in vitro and in vivo. Leukemia. 22:179–185. DOI: 10.1038/sj.leu.2404959. PMID: 17898787.
Article18. Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, et al. 2002; Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 99:754–758. DOI: 10.1182/blood.V99.3.754. PMID: 11806974.19. Weng WK, Levy R. 2003; Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J Clin Oncol. 21:3940–3947. DOI: 10.1200/JCO.2003.05.013. PMID: 12975461.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Desensitization in HLA Incompatible Transplantation
- ABO-incompatible living donor liver transplantation with a simplified desensitization and immunosuppression protocol: a single center retrospective study
- Immunologic strategies and outcomes in ABO-incompatible living donor liver transplantation
- ABO-Incompatible Living Donor Liver Transplantation
- Overcoming high pre-transplant isoagglutinin titers using high-dose intravenous immunoglobulin, salvage plasmapheresis, and booster rituximab without splenectomy in ABO-incompatible living donor liver transplantation: a case report