J Korean Med Sci.
2011 Jun;26(6):778-784. 10.3346/jkms.2011.26.6.778.
Effects of Scutellarin on MUC5AC Mucin Production Induced by Human Neutrophil Elastase or Interleukin 13 on Airway Epithelial Cells
- Affiliations
-
- 1Department of Respiratory Medicine, the Second Affiliated Hospital, Chongqing Medical University, Chongqing, China. zxd999@263.net
- 2Far Eastern Scientific Center of Physiology and Pathology of Respiration, Blagoveschensk, Russia.
- KMID: 1785964
- DOI: http://doi.org/10.3346/jkms.2011.26.6.778
Abstract
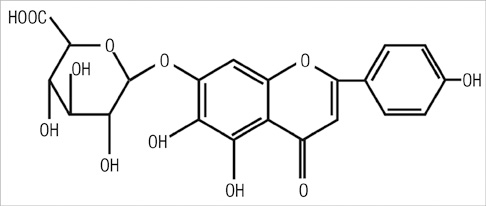
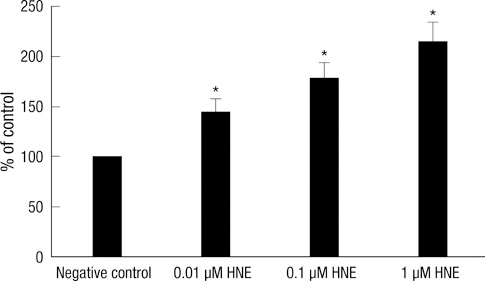
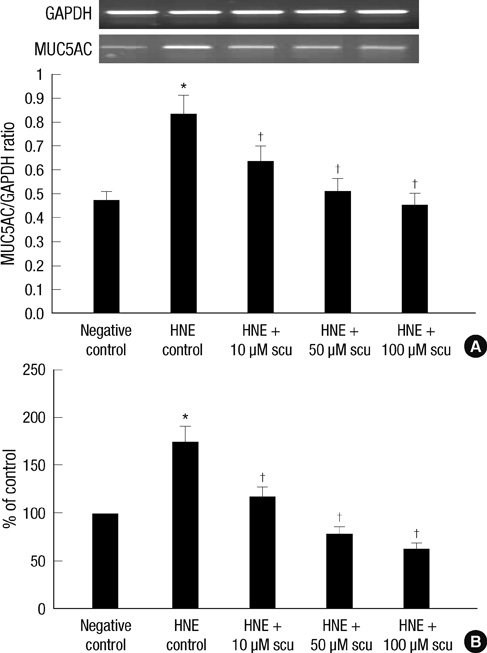
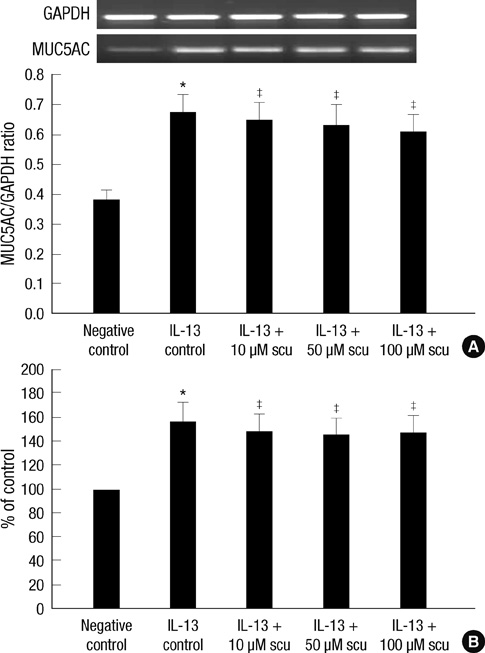
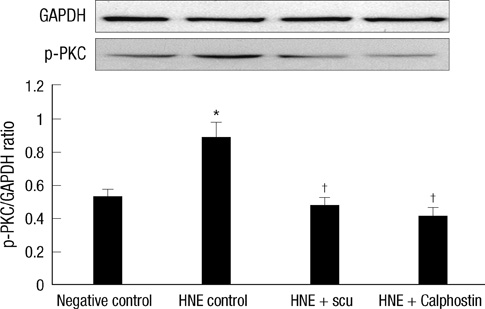
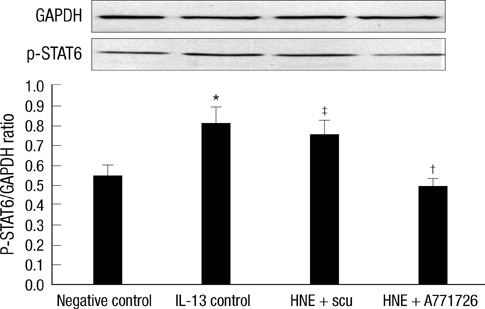
- Scutellarin is a flavonoid extracted from a traditional Chinese herb, Erigeron breviscapus. The present study investigated the effect of scutellarin on MUC5AC mucin production and the possible mechanism. Human bronchial epithelial 16 (HBE16) cells were pretreated with scutellarin for 60 min, and then exposed to human neutrophil elastase (HNE) or interleukin (IL)-13 for 12 hr. RT-PCR and ELISA were performed to measure the amount of MUC5AC mucin production. The results showed that scutellarin inhibited MUC5AC expression both in mRNA and protein level induced by HNE in a concentration-dependent manner. However, scutellarin failed to inhibit MUC5AC mucin production induced by IL-13. To investigate the intracellular mechanisms associated with the effect of scutellarin on MUC5AC mucin production, western blotting was carried out to examine the phosphorylation of protein kinase C (PKC), signal transducer and activator of transcription 6 (STAT6) and extracellular signal-regulated kinase 1/2 (ERK1/2). The phosphorylation of PKC and ERK1/2 was attenuated after treatment with scutellarin, whereas STAT6 was not significantly affected. Therefore, it is suggested that scutellarin down-regulates MUC5AC mucin production on HBE16 cells via ERK-dependent and PKC-dependent pathways.
MeSH Terms
-
Apigenin/chemistry/*pharmacology
Cells, Cultured
Dose-Response Relationship, Drug
Down-Regulation
Epithelial Cells/*drug effects/metabolism
Erigeron/chemistry
Glucuronic Acids/chemistry/*pharmacology
Humans
Interleukin-13/*pharmacology
Leukocyte Elastase/*pharmacology
Mitogen-Activated Protein Kinase 1/metabolism
Mitogen-Activated Protein Kinase 3/metabolism
Mucin 5AC/genetics/*metabolism
Phosphorylation
Protein Kinase C/metabolism
Respiratory Mucosa/drug effects/*metabolism
STAT6 Transcription Factor/metabolism
Signal Transduction
Figure
Reference
-
1. Caramori G, Di Gregorio C, Carlstedt I, Casolari P, Guzzinati I, Adcock IM, Barnes PJ, Ciaccia A, Cavallesco G, Chung KF, Papi A. Mucin expression in peripheral airways of patients with chronic obstructive pulmonary disease. Histopathology. 2004. 45:477–484.2. Young HW, Williams OW, Chandra D, Bellinghausen LK, Pérez G, Suárez A, Tuvim MJ, Roy MG, Alexander SN, Moghaddam SJ, Adachi R, Blackburn MR, Dickey BF, Evans CM. Central role of Muc5ac expression in mucous metaplasia and its regulation by conserved 5' elements. Am J Respir Cell Mol Biol. 2007. 37:273–290.3. Voynow JA, Fischer BM, Malarkey DE, Burch LH, Wong T, Longphre M, Ho SB, Foster WM. Neutrophil elastase induces mucus cell metaplasia in mouse lung. Am J Physiol Lung Cell Mol Physiol. 2004. 287:L1293–L1302.4. Shao MX, Nadel JA. Neutrophil elastase induces MUC5AC mucin production in human airway epithelial cells via a cascade involving protein kinase C, reactive oxygen species, and TNF-alpha- converting enzyme. J Immunol. 2005. 175:4009–4016.5. Tanabe T, Fujimoto K, Yasuo M, Tsushima K, Yoshida K, Ise H, Yamaya M. Modulation of mucus production by interleukin-13 receptor alpha 2 in the human airway epithelium. Clin Exp Allergy. 2008. 38:122–134.6. Shinkai M, Henke MO, Rubin BK. Macrolide antibiotics as immunomodulatory medications: proposed mechanisms of action. Pharmacol Ther. 2008. 117:393–405.7. Ram A, Das M, Ghosh B. Curcumin attenuates allergen-induced airway hyperresponsiveness in sensitized guinea pigs. Biol Pharm Bull. 2003. 26:1021–1024.8. Heo HJ, Lee SY, Lee MN, Lee HJ, Seok JH, Lee CJ. Genistein and curcumin suppress epidermal growth factor-induced MUC5AC mucin production and gene expression from human airway epithelial cells. Phytother Res. 2009. 23:1458–1461.9. Gao ZX, Huang DY, Li HX, Zhang LN, Lv YH, Cui HD, Zheng JH. Scutellarin promotes in vitro angiogenesis in human umbilical vein endotheli al cells. Biochem Biophys Res Commun. 2010. 400:151–156.10. Lin LL, Liu AJ, Liu JG, Yu XH, Qin LP, Su DF. Protective effects of scutellarin and breviscapine on brain and heart ischemia in rats. J Cardiovasc Pharmacol. 2007. 50:327–332.11. Pan Z, Zhao W, Zhang X, Wang B, Wang J, Sun X, Liu X, Feng S, Yang B, Lu Y. Scutellarin alleviates interstitial fibrosis and cardiac dysfunction of infarct rats by inhibiting TGFβ1 expression and activation of p38-MAPK and ERK1/2. Br J Pharmacol. 2011. 162:688–700.12. Xu W, Zha RP, Wang WY, Wang YP. Effects of scutellarin on PKCgamma in PC12 cell injury induced by oxygen and glucose deprivation. Acta Pharmacol Sin. 2007. 28:1573–1579.13. Wang M, Zhang WB, Zhu JH, Fu GS, Zhou BQ. Breviscapine ameliorates hypertrophy of cardiomyocytes induced by high glucose in diabetic rats via the PKC signaling pathway. Acta Pharmacol Sin. 2009. 30:1081–1091.14. Abdullah LH, Bundy JT, Ehre C, Davis CW. Mucin secretion and PKC isoforms in SPOC1 goblet cells: differential activation by purinergic agonist and PMA. Am J Physiol Lung Cell Mol Physiol. 2003. 285:L149–L160.15. Park JA, He F, Martin LD, Li Y, Chorley BN, Adler KB. Human neutrophil elastase induces hypersecretion of mucin from well-differentiated human bronchial epithelial cells in vitro via a protein kinase C{delta}-mediated mechanism. Am J Pathol. 2005. 167:651–661.16. Zhen G, Park SW, Nguyenvu LT, Rodriguez MW, Barbeau R, Paquet AC, Erle DJ. IL-13 and epidermal growth factor receptor have critical but distinct roles in epithelial cell mucin production. Am J Respir Cell Mol Biol. 2007. 36:244–253.17. Yan L, Huang H, Tang QZ, Zhu LH, Wang L, Liu C, Bian ZY, Li H. Breviscapine protects against cardiac hypertrophy through blocking PKC-alpha-dependent signaling. J Cell Biochem. 2010. 109:1158–1171.18. Whittaker L, Niu N, Temann UA, Stoddard A, Flavell RA, Ray A, Homer RJ, Cohn L. Interleukin-13 mediates a fundamental pathway for airway epithelial mucus induced by CD4 T cells and interleukin-9. Am J Respir Cell Mol Biol. 2002. 27:593–602.19. Rhee CK, Kang CM, You MB, Yoon HK, Kim YK, Kim KH, Moon HS, Park SH, Song JS. Effect of fudosteine on mucin production. Eur Respir J. 2008. 32:1195–1202.20. Kuwahara I, Lillehoj EP, Lu W, Singh IS, Isohama Y, Miyata T, Kim KC. Neutrophil elastase induces IL-8 gene transcription and protein release through p38/NF-{kappa}B activation via EGFR transactivation in a lung epithelial cell line. Am J Physiol Lung Cell Mol Physiol. 2006. 291:L407–L416.21. Zhu BH, Ma L, Pan XD, Huang YL, Liu J. Scutellarin induced Ca(2+) release and blocked KCl-induced Ca(2+) influx in smooth muscle cells isolated from rat thoracic artery. J Asian Nat Prod Res. 2008. 10:583–589.22. Pan ZW, Zhang Y, Mei DH, Zhang R, Wang JH, Zhang XY, Xu CQ, Lu YJ, Yang BF. Scutellarin exerts its anti-hypertrophic effects via suppressing the Ca2+-mediated calcineurin and CaMKII signaling pathways. Naunyn Schmiedebergs Arch Pharmacol. 2010. 381:137–145.23. Hong H, Liu GQ. Protection against hydrogen peroxide-induced cytotoxicity in PC12 cells by scutellarin. Life Sci. 2004. 74:2959–2973.24. Nakanishi A, Morita S, Iwashita H, Sagiya Y, Ashida Y, Shirafuji H, Fujisawa Y, Nishimura O, Fujino M. Role of gob-5 in mucus overproduction and airway hyperresponsiveness in asthma. Proc Natl Acad Sci USA. 2001. 98:5175–5180.25. Lu S, Liu H, Farley JM Sr. Macrolide antibiotics inhibit mucus secretion and calcium entry in swine airway submucosal mucous gland cells. J Pharmacol Exp Ther. 2011. 336:178–187.26. Wang M, Zhang WB, Zhu JH, Fu GS, Zhou BQ. Breviscapine ameliorates hypertrophy of cardiomyocytes induced by high glucose in diabetic rats via the PKC signaling pathway. Acta Pharmacol Sin. 2009. 30:1081–1091.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Curcumin and Genistein on Phorbol Ester or Tumor Necrosis Factor-alpha-Induced Mucin Production from Human Airway Epithelial Cells
- Effects of Homogentisic Acid and Natural Products Derived from Pinellia ternata on Secretion, Production and Gene Expression of MUC5AC Mucin from Cultured Airway Epithelial Cells
- Effects of Cynaroside, Cynarin and Linarin on Secretion, Production and Gene Expression of Airway MUC5AC Mucin in NCI-H292 Cells
- Eriodictyol Inhibits the Production and Gene Expression of MUC5AC Mucin via the IκBα-NF-κB p65 Signaling Pathway in Airway Epithelial Cells
- Effects of Nodakenin, Columbianadin, and Umbelliferone Isolated from the Roots of Angelica decursiva on the Gene Expression and Production of MUC5AC Mucin from Human Airway Epithelial NCI-H292 Cells