J Korean Med Sci.
2007 Sep;22(Suppl):S98-S103. 10.3346/jkms.2007.22.S.S98.
A Phase II Study of Cetuximab (Erbitux(R)) plus FOLFIRI for Irinotecan and Oxaliplatin-refractory Metastatic Colorectal Cancer
- Affiliations
-
- 1Division of Oncology, Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. ykkang@amc.seoul.kr
- KMID: 1785796
- DOI: http://doi.org/10.3346/jkms.2007.22.S.S98
Abstract
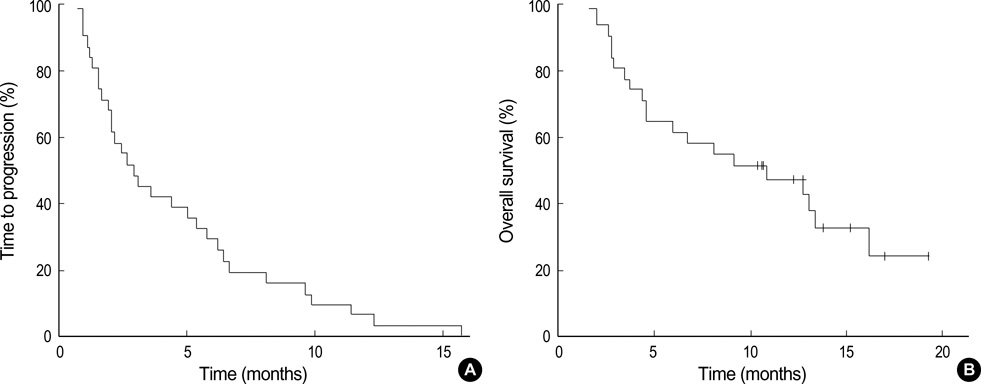
- We have evaluated the efficacy and safety of cetuximab plus FOLFIRI for irinotecan and oxaliplatin-refractory colorectal cancers. From September 2004 to February 2006, 31 patients with metastatic colorectal cancer were treated with cetuximab (400 mg/m2 intravenously [IV] over 2 hr on day 1 followed by weekly 1-hr infusions of 250 mg/m2) plus bi-weekly FOLFIRI (irinotecan 150 mg/m2 IV over 90 min, and leucovorin 100 mg/m2 IV over 2 hr, followed by 5-FU 400 mg/m2 IV bolus on day 1, and followed by 5-FU 2,400 mg/m2 by continuous IV over 46 hrs). Patients received a median of four cycles (range: 1-23). Eight (25.8%) patients had confirmed partial responses and 10 (32.2%) had stable disease. After a median follow-up of 13.2 months for surviving patients, the median time to progression was 2.9 months, the median duration of response was 5.4 months, and the median overall survival was 10.9 months. Skin toxicity was observed in 25 patients (80.4%) including grade 3 in 6 patients (19.4%). Other common non-hematologic toxicities of all grades were mucositis (32.3%), asthenia (22.6%), diarrhea (12.9%), and paronychial cracking (12.9%). The combination of cetuximab with FOLFIRI was effective and tolerable in colorectal cancer patients heavily pretreated with a number of chemotherapy regimens.
Keyword
MeSH Terms
-
Adult
Aged
Antibodies, Monoclonal/*administration & dosage/adverse effects
Antineoplastic Agents/*administration & dosage/adverse effects
Antineoplastic Combined Chemotherapy Protocols/*administration & dosage/adverse
Camptothecin/administration & dosage/adverse effects/analogs & derivatives
Colorectal Neoplasms/*drug therapy/secondary
Drug Resistance, Neoplasm
Female
Fluorouracil/administration & dosage/adverse effects
Humans
Leucovorin/administration & dosage/adverse effects
Male
Middle Aged
Organoplatinum Compounds/pharmacology
Prognosis
Receptor, Epidermal Growth Factor/antagonists & inhibitors
Safety
Figure
Reference
-
1. Shin HR, Jung KW, Won YJ, Park JG. 2002 Annual report of the Korea central cancer registry: based on registered data from 139 hospitals. Cancer Res Treat. 2004. 36:103–114.
Article2. Desch CE, Benson AB 3rd, Smith TJ, Flynn PJ, Krause C, Loprinzi CL, Minsky BD, Petrelli NJ, Pfister DG, Somerfield MR. Recommended colorectal cancer surveillance guidelines by the American Society of Clinical Oncology. J Clin Oncol. 1999. 17:1312.
Article3. Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ, Maroun JA, Ackland SP, Locker PK, Pirotta N, Elfring GL, Miller LL. Irinotecan Study Group. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. N Engl J Med. 2000. 343:905–914.
Article4. Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, Findlay BP, Pitot HC, Alberts SR. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004. 22:23–30.
Article5. Mayer A, Takimoto M, Fritz E, Schellander G, Kofler K, Ludwig H. The prognostic significance of proliferating cell nuclear antigen, epidermal growth factor receptor, and mdr gene expression in colorectal cancer. Cancer. 1993. 71:2454–2460.6. Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C, Chau I, Van Cutsem E. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004. 351:337–345.
Article7. Prewett MC, Hooper AT, Bassi R, Ellis LM, Waksal HW, Hicklin DJ. Enhanced antitumor activity of anti-epidermal growth factor receptor monoclonal antibody IMC-C225 in combination with irinotecan (CPT-11) against human colorectal tumor xenografts. Clin Cancer Res. 2002. 8:994–1003.8. Folprecht G, Lutz MP, Schoffski P, Seufferlein T, Nolting A, Pollert P, Kohne CH. Cetuximab and irinotecan/5-fluorouracil/folinic acid is a safe combination for the first-line treatment of patients with epidermal growth factor receptor expressing metastatic colorectal carcinoma. Ann Oncol. 2006. 17:450–456.
Article9. Kim ST, Kang WK, Kang JH, Park KW, Lee J, Lee SH, Park JO, Kim K, Kim WS, Jung CW, Park YS, Im YH, Park K. Salvage chemotherapy with irinotecan, 5-fluorouracil and leucovorin for taxane- and cisplatin-refractory, metastatic gastric cancer. Br J Cancer. 2005. 92:1850–1854.
Article10. Saltz LB, Meropol NJ, Loehrer PJ Sr, Needle MN, Kopit J, Mayer RJ. Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol. 2004. 22:1201–1208.
Article11. Vincenzi B, Santini D, Rabitti C, Coppola R, Beomonte Zobel B, Trodella L, Tonini G. Cetuximab and irinotecan as third-line therapy in advanced colorectal cancer patients: a single centre phase II trial. Br J Cancer. 2006. 94:792–797.
Article12. Chung KY, Shia J, Kemeny NE, Shah M, Schwartz GK, Tse A, Hamilton A, Pan D, Schrag D, Schwartz L, Klimstra DS, Fridman D, Kelsen DP, Saltz LB. Cetuximab shows activity in colorectal cancer patients with tumors that do not express the epidermal growth factor receptor by immunohistochemistry. J Clin Oncol. 2005. 23:1803–1810.
Article13. The NCCN Clinical Practice Guidelines in Oncology: Guidelines for Treatment of Cancer by Site, Colon/Rectal Cancer. 2006. accessed 29 September 2006. Available at http://nccn.org/professionals/physician_gls/PDF/colon.pdf .14. Shia J, Klimstra DS, Li AR, Qin J, Saltz L, Teruya-Feldstein J, Akram M, Chung KY, Yao D, Paty PB, Gerald W, Chen B. Epidermal growth factor receptor expression and gene amplification in colorectal carcinoma: an immunohistochemical and chromogenic in situ hybridization study. Mod Pathol. 2005. 18:1350–1356.
Article15. Vallbohmer D, Zhang W, Gordon M, Yang DY, Yun J, Press OA, Rhodes KE, Sherrod AE, Iqbal S, Danenberg KD, Groshen S, Lenz H-J. Molecular determinants of cetuximab efficacy. J Clin Oncol. 2005. 23:3536–3544.16. Alberts SR, Sinicrope FA, Grothey A. N0147: a randomized phase III trial of oxaliplatin plus 5-fluorouracil/leucovorin with or without cetuximab after curative resection of stage III colon cancer. Clin Colorectal Cancer. 2005. 5:211–213.
Article17. Meyerhardt JA, Heseltine D, Ogino S, Clark JW, Enzinger PC, Ryan DP, Earle CC, Zhu AX, Fuchs CS. Efficacy of cetuximab after treatment with oral epidermal growth factor receptor tyrosine kinase inhibitor-based chemotherapy in metastatic colorectal cancer. Clin Colorectal Cancer. 2006. 6:59–65.
Article18. Cetuximab and/or Bevacizumab Combined With Combination Chemotherapy in Treating Patients With Metastatic Colorectal Cancer. National Cancer Institute (NCI). 2006. accessed 29 September 2006. Available at http://ClinicalTrials.gov .19. Venook A, Niedzwiecki D, Hollis D, Sutherland S, Goldberg R, Alberts S, Benson A, Wade J, Schilsky R, Mayer R. Phase III study of irinotecan/5FU/LV (FOLFIRI) or oxaliplatin/5FU/LV (FOLFOX) ± cetuximab for patients (pts) with untreated metastatic adenocarcinoma of the colon or rectum (MCRC): CALGB 80203 preliminary results. J Clin Oncol. 2006. 24:3509.
Article20. Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, Ove R, Kies MS, Baselga J, Youssoufian H, Amellal N, Rowinsky EK, Ang KK. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006. 354:567–578.
Article21. Schrag D. The price tag on progress-chemotherapy for colorectal cancer. N Engl J Med. 2004. 351:317–319.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Retrospective Analysis of Patients Treated with Cetuximab plus FOLFIRI for Previous Irinotecan-combined Chemotherapy in Metastatic Colorectal Cancer
- Chemotherapy for Colorectal Cancer
- The Role of Targeted Therapy in the Treatment of Metastatic Colorectal Cancer
- Drug-induced immune-mediated thrombocytopenia due to bevacizumab-FOLFOX therapy: a case report
- Imrpoving Outcomes with Chemotherapy in Colorectal Cancer: Current Options, Current Evidence