J Korean Med Sci.
2007 Dec;22(6):1015-1021. 10.3346/jkms.2007.22.6.1015.
ERK-1/-2 and p38 Kinase Oppositely Regulate 15-deoxy-delta(12,14)-prostaglandinJ2-Induced PPAR-gamma Activation That Mediates Dedifferentiation But Not Cyclooxygenase-2 Expression in Articular Chondrocytes
- Affiliations
-
- 1Department of Biological Sciences, College of Natural Sciences, Kongju National University, Gongju, Korea. ksj85@kongju.ac.kr
- 2Laboratory of Radiation Experimental Therapeutics, Korea Institute of Radiological and Medical Sciences, Seoul, Korea.
- KMID: 1785763
- DOI: http://doi.org/10.3346/jkms.2007.22.6.1015
Abstract
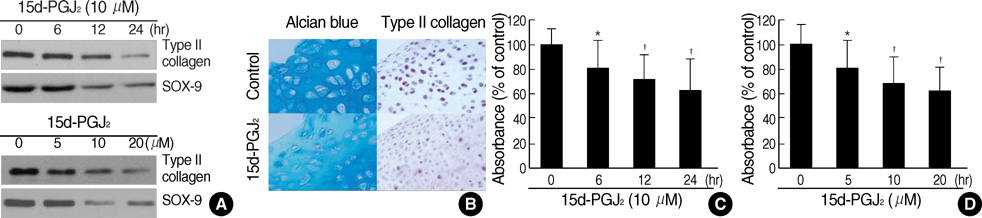
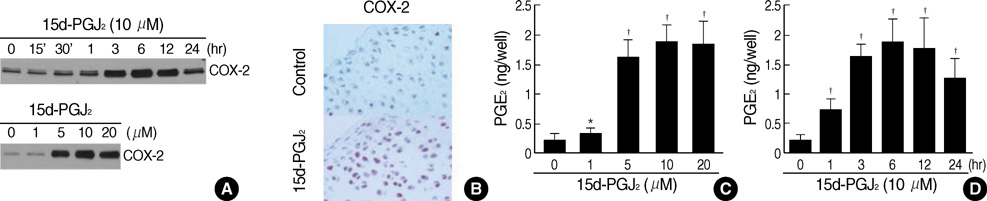
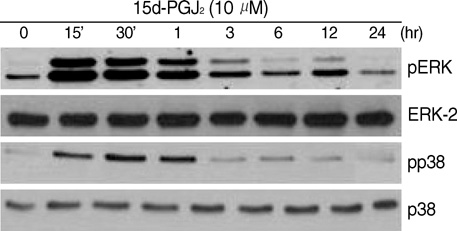
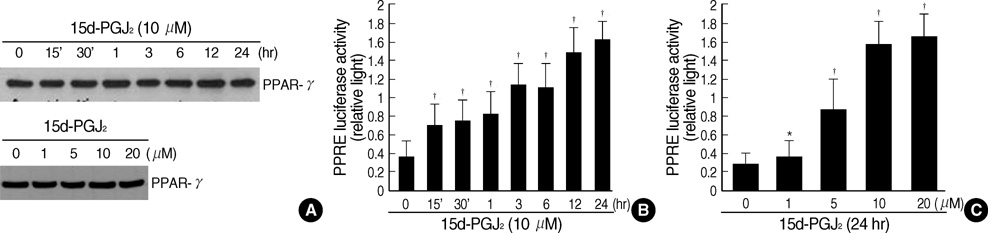
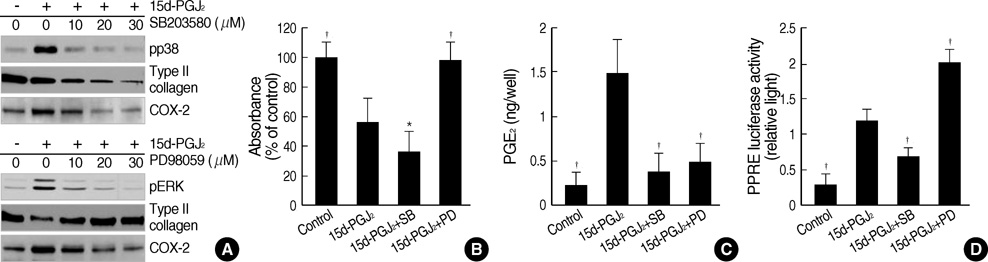
- Peroxisome proliferator-activated receptor gamma (PPAR-gamma) is a ligand-activated transcription factor and plays an important role in growth, differentiation, and inflammation in different tissues. In this study, we investigated the effects of 15d-PGJ2, a high-affinity ligand of PPAR-gamma, on dedifferentiation and on inflammatory responses such as COX-2 expression and PGE2 production in rabbit articular chondrocytes with a focus on ERK-1/-2, p38 kinase, and PPAR-gamma activation. We report here that 15d-PGJ2 induced dedifferentiation and/or COX-2 expression and subsequent PGE2 production. 15d-PGJ2 treatment stimulated activation of ERK-1/-2, p38 kinase, and PPAR-gamma. Inhibition of ERK-1/-2 with PD98059 recovered 15d-PGJ2-induced dedifferentiation and enhanced PPAR-gamma activation, whereas inhibition of p38 kinase with SB203580 potentiated dedifferentiation and partially blocked PPAR-gamma activation. Inhibition of ERK-1/-2 and p38 kinase abolished 15d-PGJ2-induced COX-2 expression and subsequent PGE2 production. Our findings collectively suggest that ERK-1/-2 and p38 kinase oppositely regulate 15d-PGJ2-induced dedifferentiation through a PPAR-gamma-dependent mechanism, whereas COX-2 expression and PGE2 production is regulated by ERK-1/-2 through a PPAR-gamma-independent mechanism but not p38 kinase in articular chondrocytes. Additionally, these data suggest that targeted modulation of the PPAR-gamma and mitogen-activated protein kinase pathway may offer a novel approach for therapeutic inhibition of joint tissue degradation.
Keyword
MeSH Terms
-
Animals
Cartilage, Articular/*cytology
Cell Differentiation/drug effects
Chondrocytes/cytology/*drug effects/metabolism
Cyclooxygenase 2/*analysis
Dinoprostone/biosynthesis
Mitogen-Activated Protein Kinase 1/*physiology
Mitogen-Activated Protein Kinase 3/*physiology
PPAR gamma/*physiology
Prostaglandin D2/*analogs & derivatives/pharmacology
Rabbits
p38 Mitogen-Activated Protein Kinases/*physiology
Figure
Cited by 1 articles
-
The Protective Effect of Sodium Hyaluronate on the Cartilage of Rabbit Osteoarthritis by Inhibiting Peroxisome Proliferator-Activated Receptor-Gamma Messenger RNA Expression
Jian-lin Zhou, Shi-qing Liu, Bo Qiu, Qiong-jie Hu, Jiang-hua Ming, Hao Peng
Yonsei Med J. 2009;50(6):832-837. doi: 10.3349/ymj.2009.50.6.832.
Reference
-
1. DeLise AM, Fischer L, Tuan RS. Cellular interactions and signaling development. Osteoarthrtis Cartilage. 2000. 8:309–334.2. Sandell LJ, Aigner T. Articular cartilage and changes in arthritis. An introduction: cell biology of osteoarthritis. Arthritis Research. 2001. 3:107–113.
Article3. Isenmann I, Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990. 347:645–650.
Article4. Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell. 1994. 79:1147–1156.5. Chawla A, Schwar EJ, Dimaculangan DD, Lazar MA. Peroxisome proliferator-activated receptor (PPAR) gamma: adipose-predominant expression and induction early in adipocyte differentiation. Endocrinology. 1994. 135:798–800.
Article6. Xin X, Yang S, Kowalski J, Gerritsen ME. Peroxisome proliferator-activated receptor gamma ligands are potent inhibitors of angiogenesis in vitro and in vivo. J Biol Chem. 1999. 274:9116–9121.7. Bordji K, Grillasca JP, Gouze JN, Magdalou J, Schohn H, Keller JM, Bianchi A, Dauca M, Netter P, Terlain B. Evidence for the presence of peroxisome proliferator-activated receptor (PPAR) alpha and gamma and retinoid Z receptor in cartilage. PPARgamma activation modulates the effects of interleukin-1beta on rat chondrocytes. J Biol Chem. 2000. 275:12243–12250.8. Fahmi H, Di Battista JA, Pelletier JP, Mineau F, Ranger P, Martel-Pelletier J. Peroxisome proliferator-activated receptor gamma activators inhibit interleukin-1beta-induced nitric oxide and matrix metalloproteinase 13 production in human chondrocytes. Arthritis Rheum. 2001. 44:595–607.9. Matsuoka T, Hirata M, Tanaka H, Takashi Y, Murata T, Kabashima K, Sugimoto Y, Kobayashi T, Ushikubi F, Aze Y, Eguhi N, Urade Y, Yoshida N, Kimura K, Mizoguchi A, Honda Y, Nagai H, Narumiya S. Prostaglandin D2 as a mediator of allergic asthma. Science. 2000. 287:2013–2017.
Article10. Cuzzocrea S, Wayman NS, Mazzon E, Dugo L, Di Paola R, Serraino I, Britti D, Chattergee PK, Caputi AP, Thiemermann C. The cyclopentenone prostaglandin 15-deoxy-Delta (12,14)-prostaglandin J(2) attenuates the development of acute and chronic inflammation. Mol Pharmacol. 2002. 61:997–1007.11. Kliewer SA, Lenhard JM, Willson TM, Patel I, Morris DC, Lehmann JM. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor gamma and promotes adipocyte differentiation. Cell. 1995. 83:813–819.12. Tugwood JD, Montague CT. Biology and toxicology of PPARgamma ligands. Hum Exp Toxicol. 2002. 21:429–437.13. Bishop-Bailey D, Hla T. Endothelial cell apoptosis induced by the peroxisome proliferator-activated receptor (PPAR) ligand 15-deoxy-Delta12, 14-prostaglandin J2. J Biol Chem. 1999. 274:17042–17048.14. Kim SJ, Kim HG, Oh CD, Hwang SG, Song WK, Yoo YJ, Kang SS, Chun JS. p38 kinase-dependent and -independent Inhibition of protein kinase C zeta and -alpha regulates nitric oxide-induced apoptosis and dedifferentiation of articular chondrocytes. J Biol Chem. 2002. 277:30375–30381.15. Camp HS, Tafuri SR, Leff T. c-Jun N-terminal kinase phosphorylates peroxisome proliferator-activated receptor-gamma1 and negatively regulates its transcriptional activity. Endocrinology. 1999. 140:392–397.16. Rossi A, Kapahi P, Natoli G, Takahashi T, Chen Y, Karin M, Santoro MG. Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of IkappaB kinase. Nature. 2000. 403:103–108.17. Maggi LB, Sadeghi H, Weigand C, Scarim AL, Heitmeier MR, Corbett JA. Anti-inflammatory actions of 15-deoxy-delta 12,14-prostaglandin J2 and troglitazone: evidence for heat shock-dependent and -independent inhibition of cytokine-induced inducible nitric oxide synthase expression. Diabetes. 2000. 49:346–355.
Article18. Hortelano S, Castrillo A, Alvarez AM, Bosca L. Contribution of cyclopentenone prostaglandins to the resolution of inflammation through the potentiation of apoptosis in activated macrophages. J Immunol. 2000. 165:6525–6531.
Article19. Yoon YM, Kim SJ, Oh CD, Ju JW, Song WK, Yoo YJ, Huh TL, Chun JS. Maintenance of differentiated phenotype of articular chondrocytes by protein kinase C and extracellular signal-regulated protein kinase. J Biol Chem. 2002. 277:8412–8420.
Article20. Oh CD, Chang SH, Yoon YM, Lee SJ, Lee YS, Kang SS, Chun JS. Opposing role of mitogen-activated protein kinase subtypes, ERK-1/2 and p38, in the regulation of chondrogenesis of mesenchymes. J Biol Chem. 2000. 275:5613–5619.
Article21. Poole AR. Mc Carthy DJ, Koopman WJ, editors. Arthritis and Allied Conditions. 2003. 12th Ed.Philadelphia: Lea and Febiger;279–333.22. Goldring MB, Birkhead J, Sandell LJ, Kimura T, Krane SM. Interleukin 1 suppresses expression of cartilage-specific types II and IX collagens and increases types I and III collagens in human chondrocytes. J Clin Invest. 1988. 82:2026–2037.
Article23. Ryu JH, Kim SJ, Kim SH, Oh CD, Hwang SG, Chun CH, Oh SH, Seong JK, Huh TL, Chun JS. Regulation of the chondrocyte phenotype by beta-catenin. Development. 2002. 129:5541–5550.
Article24. Kim SJ, Ju JW, Oh CD, Yoon YM, Song WK, Kim JH, Yoo YJ, Bang OS, Kang SS, Chun JS. ERK-1/2 and p38 kinase oppositely regulate nitric oxide-induced apoptosis of chondrocytes in association with p53, caspase-3, and differentiation status. J Biol Chem. 2002. 277:1332–1339.
Article25. Benya PD, Padilla SR, Nimni ME. Independent regulation of collagen types by chondrocytes during the loss of differentiated function in culture. Cell. 1978. 15:1313–1321.
Article26. Gay S, Gay RE, Koopman WJ. Molecular and cellular mechanisms of joint destruction in rheumatoid arthritis: two cellular mechanisms explain joint destruction? Ann Rheum Dis. 1993. 52:Suppl 1. S39–S47.
Article27. Myers LK, Kang AH, Postlethwaite AE, Rosloniec EF, Morham SG, Shlopov , Goorha S, Ballou LR. The genetic ablation of cyclooxygenase 2 prevents the development of autoimmune arthritis. Arthritis Rheum. 2000. 43:2687–2693.
Article28. Sheng H, Williams CS, Shao J, Liang P, DuBois RN, Beauchamp RD. Induction of cyclooxygenase-2 by activated Ha-ras oncogene in Rat-1 fibroblasts and the role of mitogen-activated protein kinase pathway. J Biol Chem. 1998. 273:22120–22127.29. Faour WH, He Y, He QW, de ladurantaye M, Quintero M, Mancini A, Di Battista JA. Prostaglandin E(2) regulates the level and stability of cyclooxygenase-2 mRNA through activation of p38 mitogen-activated protein kinase in interleukin-1 beta-treated human synovial fibroblasts. J Biol Chem. 2001. 276:31720–31731.30. Matsuura H, Sakaue M, Subbaramaiah K, Kamitani H, Eling TE, Dannenberg AJ, Tanabe T, Inoue H, Arata J, Jetten AM. Regulation of cyclooxygenase-2 by interferon gamma and transforming growth factor alpha in normal human epidermal keratinocytes and squamous carcinoma cells. Role of mitogen-activated protein kinases. J Biol Chem. 1999. 274:29138–29148.31. Guan Z, Buckman SY, Miller BW, Springer LD, Morrison AR. Interleukin-1beta-induced cyclooxygenase-2 expression requires activation of both c-Jun NH2-terminal kinase and p38 MAPK signal pathways in rat renal mesangial cells. J Biol Chem. 1998. 273:28670–28676.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- 15-Deoxy-delta 12,14-ProstaglandinJ2 Regulates Dedifferentiation through Peroxisome Proliferator-Activated Receptor-gamma-Dependent Pathway but Not COX-2 Expression in Articular Chondrocytes
- Src Kinase Regulates Nitric Oxide-induced Dedifferentiation and Cyclooxygenase-2 Expression in Articular Chondrocytes via p38 Kinase-dependent Pathway
- p38 Kinase Regulates Nitric Oxide-induced Dedifferentiation and Cyclooxygenase-2 Expression of Articular Chondrocytes
- Ectopic Expression of Caveolin-1 Induces COX-2 Expression in Rabbit Articular Chondrocytes via MAP Kinase Pathway
- 15-Deoxy-Delta(12,14)-Prostaglandin J2 Upregulates the Expression of LPS-Induced IL-8/CXCL8 mRNA in Vascular Smooth Muscle Cells from Spontaneously Hypertensive Rats