J Periodontal Implant Sci.
2011 Oct;41(5):234-241. 10.5051/jpis.2011.41.5.234.
The effect of erbium-doped: yttrium, aluminium and garnet laser irradiation on the surface microstructure and roughness of double acid-etched implants
- Affiliations
-
- 1Department of Periodontology, Kyung Hee University School of Dentistry, Seoul, Korea. kyhyuk@khu.ac.kr
- 2Institute of Oral Biology, Kyung Hee University School of Dentistry, Seoul, Korea.
- KMID: 1783618
- DOI: http://doi.org/10.5051/jpis.2011.41.5.234
Abstract
- PURPOSE
One of the most frequent complications related to dental implants is peri-implantitis, and the characteristics of implant surfaces are closely related to the progression and resolution of inflammation. Therefore, a technical modality that can effectively detoxify the implant surface without modification to the surface is needed. The purpose of this study was to evaluate the effect of erbium-doped: yttrium, aluminium and garnet (Er:YAG) laser irradiation on the microstructural changes in double acid-etched implant surfaces according to the laser energy and the application duration.
METHODS
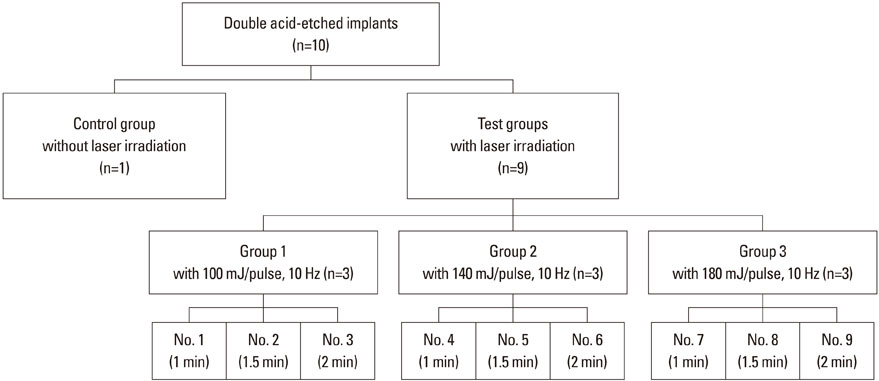
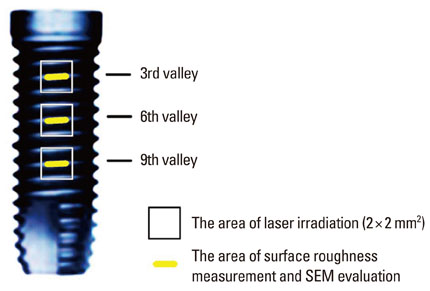
The implant surface was irradiated using an Er:YAG laser with different application energy levels (100 mJ/pulse, 140 mJ/pulse, and 180 mJ/pulse) and time periods (1 minute, 1.5 minutes, and 2 minutes). We then examined the change in surface roughness value and microstructure.
RESULTS
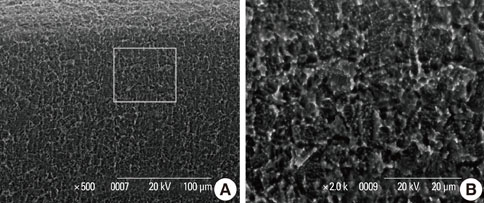
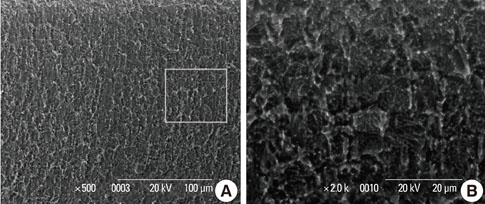
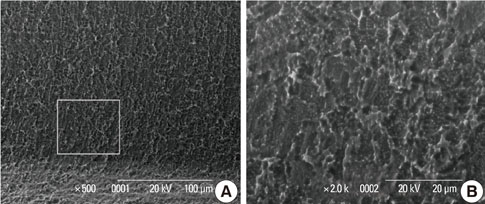
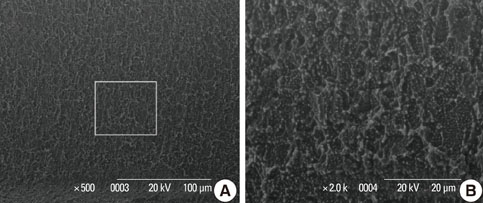
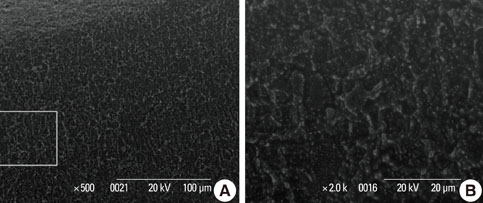
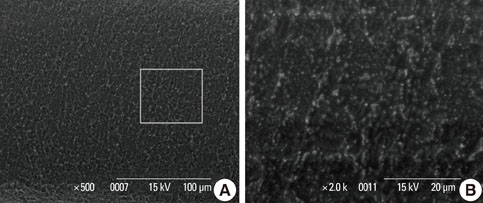
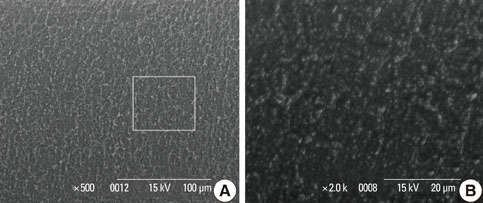
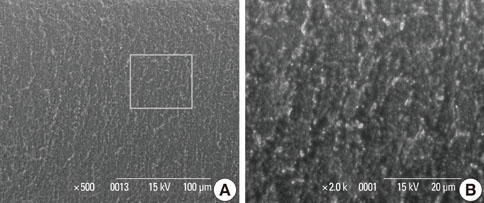
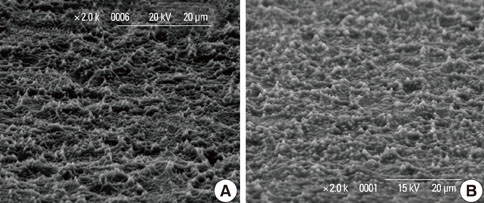
In a scanning electron microscopy evaluation, the double acid-etched implant surface was not altered by Er:YAG laser irradiation under the condition of 100 mJ/pulse at 10 Hz for any of the irradiation times. However, we investigated the reduced sharpness of the specific ridge microstructure that resulted under the 140 mJ/pulse and 180 mJ/pulse conditions. The reduction in sharpness became more severe as laser energy and application duration increased. In the roughness measurement, the double acid-etched implants showed a low roughness value on the valley area before the laser irradiation. Under all experimental conditions, Er:YAG laser irradiation led to a minor decrease in surface roughness, which was not statistically significant.
CONCLUSIONS
The recommended application settings for Er:YAG laser irradiation on double acid-etched implant surface is less than a 100 mJ/pulse at 10 Hz, and for less than two minutes in order to detoxify the implant surface without causing surface modification.
Keyword
MeSH Terms
Figure
Reference
-
1. Albrektsson TO, Johansson CB, Sennerby L. Biological aspects of implant dentistry: osseointegration. Periodontol 2000. 1994. 4:58–73.
Article2. Mombelli A, Lang NP. The diagnosis and treatment of peri-implantitis. Periodontol 2000. 1998. 17:63–76.
Article3. Quirynen M, Naert I, van Steenberghe D, Nys L. A study of 589 consecutive implants supporting complete fixed prostheses. Part I: Periodontal aspects. J Prosthet Dent. 1992. 68:655–663.
Article4. Isidor F. Loss of osseointegration caused by occlusal load of oral implants. A clinical and radiographic study in monkeys. Clin Oral Implants Res. 1996. 7:143–152.
Article5. Pongnarisorn NJ, Gemmell E, Tan AE, Henry PJ, Marshall RI, Seymour GJ. Inflammation associated with implants with different surface types. Clin Oral Implants Res. 2007. 18:114–125.
Article6. Meffert RM, Langer B, Fritz ME. Dental implants: a review. J Periodontol. 1992. 63:859–870.
Article7. Mombelli A. Microbiology and antimicrobial therapy of peri-implantitis. Periodontol 2000. 2002. 28:177–189.
Article8. Krozer A, Hall J, Ericsson I. Chemical treatment of machined titanium surfaces. An in vitro study. Clin Oral Implants Res. 1999. 10:204–211.
Article9. Schwarz F, Putz N, Georg T, Reich E. Effect of an Er:YAG laser on periodontally involved root surfaces: an in vivo and in vitro SEM comparison. Lasers Surg Med. 2001. 29:328–335.
Article10. Schwarz F, Sculean A, Berakdar M, Szathmari L, Georg T, Becker J. In vivo and in vitro effects of an Er:YAG laser, a GaAlAs diode laser, and scaling and root planing on periodontally diseased root surfaces: a comparative histologic study. Lasers Surg Med. 2003. 32:359–366.
Article11. Ando Y, Aoki A, Watanabe H, Ishikawa I. Bactericidal effect of erbium YAG laser on periodontopathic bacteria. Lasers Surg Med. 1996. 19:190–200.
Article12. Yamaguchi H, Kobayashi K, Osada R, Sakuraba E, Nomura T, Arai T, et al. Effects of irradiation of an erbium:YAG laser on root surfaces. J Periodontol. 1997. 68:1151–1155.13. Matsuyama T, Aoki A, Oda S, Yoneyama T, Ishikawa I. Effects of the Er:YAG laser irradiation on titanium implant materials and contaminated implant abutment surfaces. J Clin Laser Med Surg. 2003. 21:7–17.
Article14. Albrektsson T, Wennerberg A. Oral implant surfaces: Part 1--review focusing on topographic and chemical properties of different surfaces and in vivo responses to them. Int J Prosthodont. 2004. 17:536–543.15. De Leonardis D, Garg AK, Pecora GE. Osseointegration of rough acid-etched titanium implants: 5-year follow-up of 100 minimatic implants. Int J Oral Maxillofac Implants. 1999. 14:384–391.16. Wennerberg A, Ektessabi A, Albrektsson T, Johansson C, Andersson B. A 1-year follow-up of implants of differing surface roughness placed in rabbit bone. Int J Oral Maxillofac Implants. 1997. 12:486–494.17. Lazzara RJ, Testori T, Trisi P, Porter SS, Weinstein RL. A human histologic analysis of osseotite and machined surfaces using implants with 2 opposing surfaces. Int J Periodontics Restorative Dent. 1999. 19:117–129.18. London RM, Roberts FA, Baker DA, Rohrer MD, O'Neal RB. Histologic comparison of a thermal dual-etched implant surface to machined, TPS, and HA surfaces: bone contact in vivo in rabbits. Int J Oral Maxillofac Implants. 2002. 17:369–376.19. Park JY, Davies JE. Red blood cell and platelet interactions with titanium implant surfaces. Clin Oral Implants Res. 2000. 11:530–539.
Article20. Bowers KT, Keller JC, Randolph BA, Wick DG, Michaels CM. Optimization of surface micromorphology for enhanced osteoblast responses in vitro. Int J Oral Maxillofac Implants. 1992. 7:302–310.21. Ivanoff CJ, Widmark G, Johansson C, Wennerberg A. Histologic evaluation of bone response to oxidized and turned titanium micro-implants in human jawbone. Int J Oral Maxillofac Implants. 2003. 18:341–348.22. Hsu SH, Liu BS, Lin WH, Chiang HC, Huang SC, Cheng SS. Characterization and biocompatibility of a titanium dental implant with a laser irradiated and dual-acid etched surface. Biomed Mater Eng. 2007. 17:53–68.23. Trisi P, Rao W, Rebaudi A. A histometric comparison of smooth and rough titanium implants in human low-density jawbone. Int J Oral Maxillofac Implants. 1999. 14:689–698.24. Grossner-Schreiber B, Teichmann J, Hannig M, Dorfer C, Wenderoth DF, Ott SJ. Modified implant surfaces show different biofilm compositions under in vivo conditions. Clin Oral Implants Res. 2009. 20:817–826.25. Drake DR, Paul J, Keller JC. Primary bacterial colonization of implant surfaces. Int J Oral Maxillofac Implants. 1999. 14:226–232.26. Renvert S, Roos-Jansaker AM, Claffey N. Non-surgical treatment of peri-implant mucositis and peri-implantitis: a literature review. J Clin Periodontol. 2008. 35:8 Suppl. 305–315.
Article27. Maximo MB, de Mendonca AC, Renata Santos V, Figueiredo LC, Feres M, Duarte PM. Short-term clinical and microbiological evaluations of peri-implant diseases before and after mechanical anti-infective therapies. Clin Oral Implants Res. 2009. 20:99–108.
Article28. Kreisler M, Gotz H, Duschner H. Effect of Nd:YAG, Ho: YAG, Er:YAG, CO2, and GaAIAs laser irradiation on surface properties of endosseous dental implants. Int J Oral Maxillofac Implants. 2002. 17:202–211.29. Kreisler M, Kohnen W, Marinello C, Gotz H, Duschner H, Jansen B, et al. Bactericidal effect of the Er:YAG laser on dental implant surfaces: an in vitro study. J Periodontol. 2002. 73:1292–1298.
Article30. Folwaczny M, Mehl A, Aggstaller H, Hickel R. Antimicrobial effects of 2.94 microm Er:YAG laser radiation on root surfaces: an in vitro study. J Clin Periodontol. 2002. 29:73–78.
Article31. Friedmann A, Antic L, Bernimoulin JP, Purucker P. In vitro attachment of osteoblasts on contaminated rough titanium surfaces treated by Er:YAG laser. J Biomed Mater Res A. 2006. 79:53–60.
Article32. Schwarz F, Aoki A, Sculean A, Georg T, Scherbaum W, Becker J. In vivo effects of an Er:YAG laser, an ultrasonic system and scaling and root planing on the biocompatibility of periodontally diseased root surfaces in cultures of human PDL fibroblasts. Lasers Surg Med. 2003. 33:140–147.
Article33. Kreisler M, Kohnen W, Christoffers AB, Gotz H, Jansen B, Duschner H, et al. In vitro evaluation of the biocompatibility of contaminated implant surfaces treated with an Er: YAG laser and an air powder system. Clin Oral Implants Res. 2005. 16:36–43.
Article34. Abrahamsson I, Zitzmann NU, Berglundh T, Wennerberg A, Lindhe J. Bone and soft tissue integration to titanium implants with different surface topography: an experimental study in the dog. Int J Oral Maxillofac Implants. 2001. 16:323–332.35. Sul YT, Johansson C, Albrektsson T. Which surface properties enhance bone response to implants? Comparison of oxidized magnesium, TiUnite, and Osseotite implant surfaces. Int J Prosthodont. 2006. 19:319–328.36. Sul YT, Byon E, Wennerberg A. Surface characteristics of electrochemically oxidized implants and acid-etched implants: surface chemistry, morphology, pore configurations, oxide thickness, crystal structure, and roughness. Int J Oral Maxillofac Implants. 2008. 23:631–640.37. Lang NP, Jepsen S. Working Group 4. Implant surfaces and design (Working Group 4). Clin Oral Implants Res. 2009. 20:Suppl 4. 228–231.
Article38. Wennerberg A, Albrektsson T, Andersson B. Bone tissue response to commercially pure titanium implants blasted with fine and coarse particles of aluminum oxide. Int J Oral Maxillofac Implants. 1996. 11:38–45.39. Jeong DM, Park JB, Kwon YH, Herr Y, Chung JH. The influence of tetracycline-HCl for micromorphology of thermal dual acid etched surface implants. J Korean Acad Periodontol. 2007. 37:265–275.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of erbium-doped: yttrium, aluminium and garnet laser irradiation on the surface microstructure and roughness of sand-blasted, large grit, acid-etched implants
- The effect of Er:YAG laser irradiation on the surface microstructure and roughness of hydroxyapatite-coated implant
- Comparison of the effect of hand instruments, an ultrasonic scaler, and an erbium-doped yttrium aluminium garnet laser on root surface roughness of teeth with periodontitis: a profilometer study
- Microshear bond strength according to dentin cleansing methods before recementation
- A comparative evaluation of CO2 and erbium-doped yttrium aluminium garnet laser therapy in the management of dentin hypersensitivity and assessment of mineral content