J Korean Med Sci.
2006 Oct;21(5):891-896. 10.3346/jkms.2006.21.5.891.
Expression of the RERG Gene is Gender-Dependent in Hepatocellular Carcinoma and Regulated by Histone Deacetyltransferases
- Affiliations
-
- 1Laboratory of Human Genomics, Korea Research Institute of Bioscience and Biotechnology (KRIBB), Daejeon, Korea. dslee@kangwon.ac.kr
- 2Department of Internal Medicine, Kangnam St. Mary's Hospital, The Catholic University of Korea, Seoul, Korea.
- 3Laboratory of Molecular Oncology, Korea Institute of Radiological and Medical Sciences, Seoul, Korea.
- 4Depatment of Surgery, School of Medicine Ajou University, Suwon, Korea.
- 5Department of Pathology, College of Medicine, Chungnam National University, Daejeon, Korea.
- 6Department of Surgery, SUN General Hospital, Daejeon, Korea.
- 7Animal Resources Science, Kangwon National University, Chuncheon, Korea.
- KMID: 1781911
- DOI: http://doi.org/10.3346/jkms.2006.21.5.891
Abstract
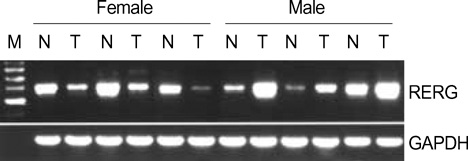
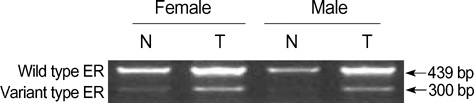
- Ras-related, estrogen-regulated, and growth-inhibitory gene (RERG) is a novel gene that was first reported in breast cancer. However, the functions of RERG are largely unknown in other tumor types. In this study, RERG expression was analyzed in hepatocellular carcinomas of human patients using reverse transcriptase PCR analysis. In addition, the possible regulation of RERG expression by histone deacetyltransferases (HDACs) was studied in several cell lines. Interestingly, the expression of RERG gene was increased in hepatocellular carcinoma (HCC) of male patients (57.9%) but decreased in HCC of females (87.5%) comparison with paired peri-tumoral tissues. Moreover, RERG gene expression was increased in murine hepatoma Hepa1-6 cells, human breast tumor MDA-MB-231 cells, and mouse normal fibroblast NIH3T3 cells after treated by HDAC inhibitor, trichostatin A. Our results suggest that RERG may function in a gender-dependent manner in hepatic tumorigenesis and that the expression of this gene may be regulated by an HDAC-related signaling pathway.
Keyword
MeSH Terms
-
Signal Transduction
Sex Factors
Mice, Transgenic
Mice, Inbred C57BL
Mice
Male
Liver Neoplasms/*genetics
Humans
Histone Deacetylases/*physiology
Hepatocytes/metabolism
Growth Inhibitors/*genetics
*Genes, ras
*Gene Expression Regulation, Neoplastic
Female
Estrogens/*pharmacology
Estrogen Receptor alpha/analysis
Cell Proliferation
Animals
Figure
Cited by 1 articles
-
Identification of genes underlying different methylation profiles in refractory anemia with excess blast and refractory cytopenia with multilineage dysplasia in myelodysplastic syndrome
Suee Lee, Hyuk-Chan Kwon, Sung-Hyun Kim, Sung Yong Oh, Ji Hyun Lee, Yeon-Su Lee, Daekwan Seo, Jin-Yeong Han, Hyo-Jin Kim
Korean J Hematol. 2012;47(3):186-193. doi: 10.5045/kjh.2012.47.3.186.
Reference
-
1. Finlin BS, Gau CL, Murphy GA, Shao H, Kimel T, Seitz RS, Chiu YF, Botstein D, Brown PO, Der CJ, Tamanoi F, Andres DA, Perou CM. RERG is a novel ras-related, estrogen-regulated and growth-inhibitory gene in breast cancer. J Biol Chem. 2001. 276:42259–42267.
Article2. Joo M, Chi JG, Lee H. Expressions of HSP70 and HSP27 in hepatocellular carcinoma. J Korean Med Sci. 2005. 20:829–834.
Article3. Kim YS, Um SH, Ryu HS, Lee JB, Lee JW, Park DK, Kim YS, Jin YT, Chun HJ, Lee HS, Lee SW, Choi JH, Kim CD, Hyun JH. The prognosis of liver cirrhosis in recent years in Korea. J Korean Med Sci. 2003. 18:833–841.
Article4. Tanaka K, Sakai H, Hashizume M, Hirohata T. Serum testosterone: estradiol ratio and the development of hepatocellular carcinoma among male cirrhotic patients. Cancer Res. 2000. 60:5106–5110.5. Firminger HI, Reuber MD. Influence of adrenocortical, androgenic, and anabolic hormones on the development of carcinoma and cirrhosis of the liver in A x C rats fed N-2-fluorenyldicetamide. J Natl Cancer Inst. 1961. 27:559–595.6. Kemp CJ, Leary CN, Drinkwater NR. Promotion of murine hepatocarcinogenesis by testosterone is androgen receptor-dependent but not cell autonomous. Proc Natl Acad Sci USA. 1989. 86:7505–7509.
Article7. Okuda K. Hepatocellular carcinoma. J Hepatol. 2000. 32:225–237.
Article8. Gray SG, Teh BT. Histone acetylation/deacetylation and cancer: an "open" and "shut" case? Curr Mol Med. 2001. 1:401–429.9. Gui CY, Ngo L, Xu WS, Richon VM, Marks PA. Histone deacetylase (HDAC) inhibitor activation of p21WAF1 involves changes in promoter-associated proteins, including HDAC1. Proc Natl Acad Sci USA. 2004. 101:1241–1246.
Article10. de Ruijter AJ, van Gennip AH, Caron HN, Kemp S, van Kuilenburg AB. Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem J. 2003. 370(Pt 3):737–749.11. Greene AK, Puder M. Partial hepatectomy in the mouse: technique and perioperative management. J Invest Surg. 2003. 16:99–102.
Article12. Villa E, Camellini L, Dugani A, Zucchi F, Grottola A, Merighi A, Buttafoco P, Losi L, Manenti F. Variant estrogen receptor messenger RNA species detected in human primary hepatocellular carcinoma. Cancer Res. 1995. 55:498–500.13. Villa E, Grottola A, Colantoni A, De Maria N, Buttafoco P, Ferretti I, Manenti F. Hepatocellular carcinoma: role of estrogen receptors in the liver. Ann N Y Acad Sci. 2002. 963:37–45.14. Wang AG, Moon HB, Lee MR, Hwang CY, Kwon KS, Yu SL, Kim YS, Kim M, Kim JM, Kim SK, Lee TH, Moon EY, Lee DS, Yu DY. Gender-dependent hepatic alterations in H-ras12V transgenic mice. J Hepatol. 2005. 43:836–844.
Article15. Alao JP, Lam EW, Ali S, Buluwela L, Bordogna W, Lockey P, Varshochi R, Stavropoulou AV, Coombes RC, Vigushin DM. Histone deacetylase inhibitor trichostatin A represses estrogen receptor alpha-dependent transcription and promotes proteasomal degradation of cyclin D1 in human breast carcinoma cell lines. Clin Cancer Res. 2004. 10:8094–8104.16. Herold C, Ganslmayer M, Ocker M, Hermann M, Geerts A, Hahn EG, Schuppan D. The histone-deacetylase inhibitor Trichostatin A blocks proliferation and triggers apoptotic programs in hepatoma cells. J Hepatol. 2002. 36:233–240.
Article17. Johnson PJ. Sex hormones and the liver. Clin Sci (Lond). 1984. 66:369–376.
Article18. Eagon PK, Porter LE, Francavilla A, DiLeo A, Van Thiel DH. Estrogen and androgen receptors in liver: their role in liver disease and regeneration. Semin Liver Dis. 1985. 5:59–69.
Article19. Roy AK, Milin BS, McMinn DM. Androgen receptor in rat liver: hormonal and developmental regulation of the cytoplasmic receptor and its correlation with the androgen-dependent synthesis of alpha 2u-globulin. Biochim Biophys Acta. 1974. 354:213–232.20. Sato N, Ota M, Obara K. Presence of binding component(s) for testosterone in rat liver cytosol. Endocrinol Jpn. 1980. 27:315–319.
Article21. Nagasue N, Ito A, Yukaya H, Ogawa Y. Androgen receptors in hepatocellular carcinoma and surrounding parenchyma. Gastroenterology. 1985. 89:643–647.
Article22. Porter LE, Elm MS, Van Thiel DH, Dugas MC, Eagon PK. Characterization and quantitation of human hepatic estrogen receptor. Gastroenterology. 1983. 84:704–712.
Article23. Rossini GP, Baldini GM, Villa E, Manenti F. Characterization of estrogen receptor from human liver. Gastroenterology. 1989. 96:1102–1109.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Functional Link between DNA Damage Responses and Transcriptional Regulation by ATM in Response to a Histone Deacetylase Inhibitor TSA
- Histone Deacetylases and Their Regulatory MicroRNAs in Hepatocarcinogenesis
- The relationship of the activation of ras oncogene to hepatitis Bviruss gene expression in human hepatocellular carcinoma
- Activation of ATM-dependent DNA Damage Signal Pathway by a Histone Deacetylase Inhibitor, Trichostatin A
- Isoproterenol increases histone deacetylase 6 expression and cell migration by inhibiting ERK signaling via PKA and Epac pathways in human lung cancer cells