Korean J Lab Med.
2008 Oct;28(5):392-399. 10.3343/kjlm.2008.28.5.392.
Availability of Fine Needle Aspirates for the Assessment of HER2 Gene Amplification in Invasive Breast Cancer Patients
- Affiliations
-
- 1Department of Laboratory Medicine, Korea Cancer Center Hospital, Seoul, Korea. jklee@kcch.re.kr
- 2Department of Surgery, Korea Cancer Center Hospital, Seoul, Korea.
- 3Department of Pathology, Korea Cancer Center Hospital, Seoul, Korea.
- KMID: 1781572
- DOI: http://doi.org/10.3343/kjlm.2008.28.5.392
Abstract
-
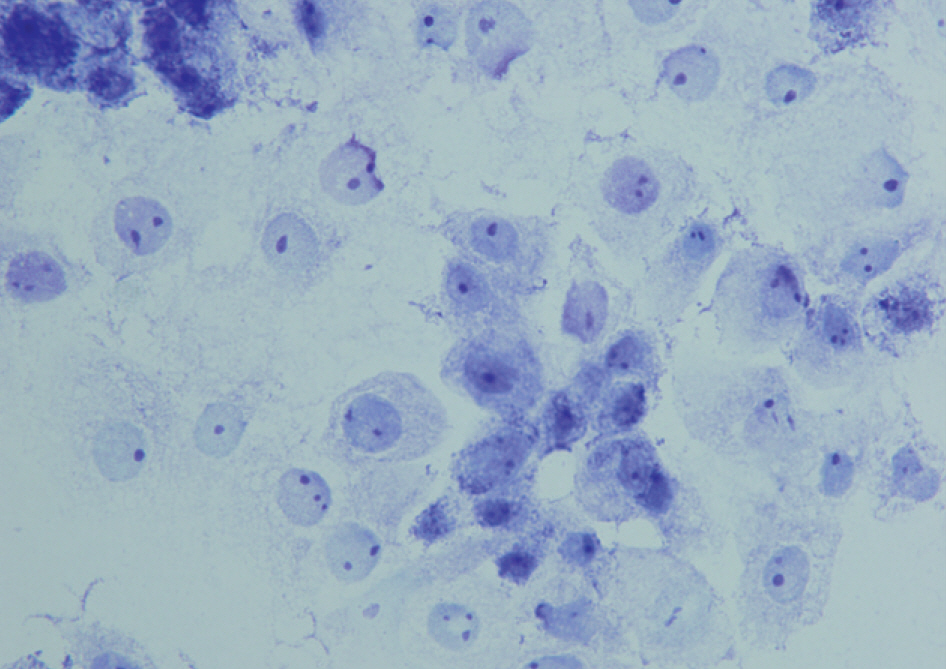
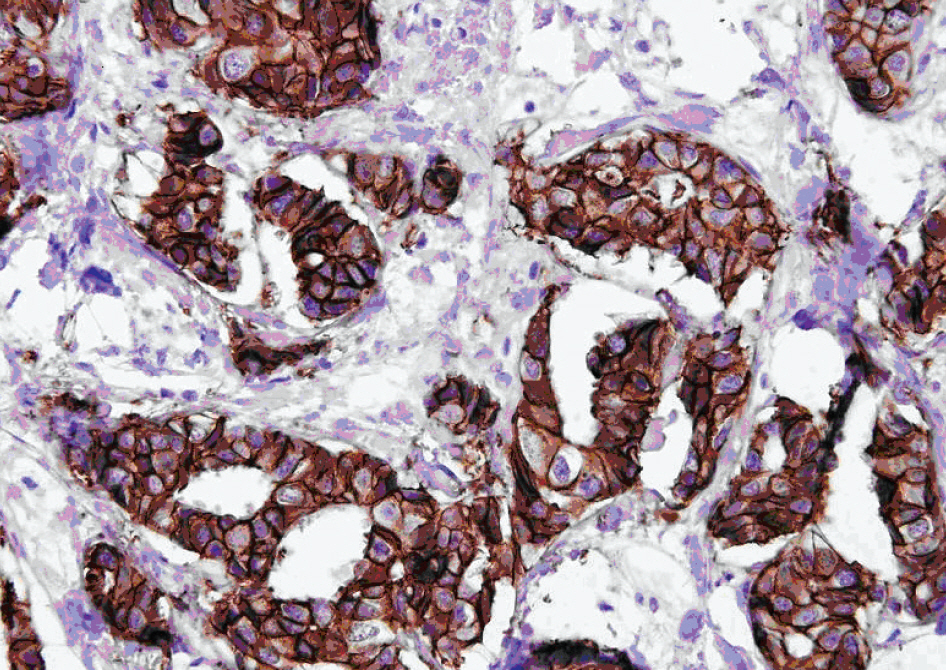
BACKGROUND: FISH and immunohistochemistry (IHC) on formalin-fixed paraffin embedded (FFPE) tissue are currently used in the clinical laboratory to determine HER2 status in invasive breast cancer patients. Since tissue-based methods are relatively time-consuming and have a limitation for standardization of procedure, we evaluated the availability of fine needle aspirates (FNA) for the assessment of HER2 status in invasive breast cancer patients.
METHODS
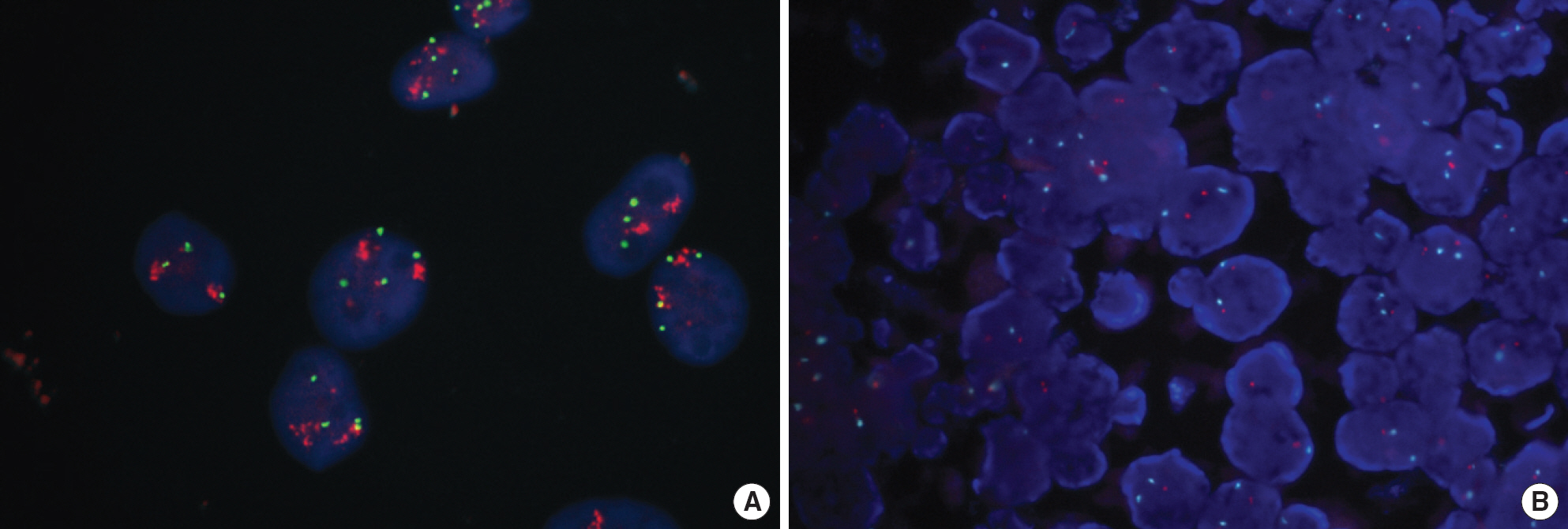
FNA were obtained from 51 invasive breast cancer patients and were submitted for the evaluation of HER2 status. After invasive breast cancer components were ascertained by morphological evaluation, HER2 gene amplification was evaluated by FISH. The results of HER2 FISH on FNA cells were compared with those of both FISH and IHC on corresponding FFPE tissues. FISH results were interpreted by American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP) guidelines issued in 2007.
RESULTS
Of 51 FNA specimens, one was excluded due to an insufficient number of cancer cells for tests. Excluding the cases that showed 'equivocal' results, 47 (98%) out of 48 cases were concordant between the results of FISH on FNA and FISH on corresponding FFPE tissue (kappa, 0.969), and 43 (93%) out of 46 cases were concordant between the results of FISH on FNA and IHC on corresponding FFPE tissue (kappa, 0.912).
CONCLUSIONS
An excellent correlation was found between FISH on FNA cells and corresponding FFPE sections. We recommend FNA specimens for more rapid determination of HER2 status by FISH, which will be helpful for patient selection for individualized therapy.
Keyword
MeSH Terms
-
Adult
Aged
Aged, 80 and over
Biopsy, Fine-Needle
Breast Neoplasms/genetics/metabolism/*pathology
Carcinoma, Ductal, Breast/genetics/metabolism/pathology
Female
Gene Amplification
Humans
Immunohistochemistry
In Situ Hybridization, Fluorescence
Middle Aged
Paraffin Embedding
Receptor, erbB-2/*genetics/metabolism
Reproducibility of Results
Figure
Cited by 1 articles
-
The Effectiveness of Silver In Situ Hybridization in Patients with Breast Cancer: A Systematic Review
Sunyoung Jang, Seon-Heui Lee, Soojin Kim, You-Kyoung Lee, Young-Hyuck Im, Wonshik Han, Hee-Sook Park
J Breast Cancer. 2011;14(Suppl 1):S1-S9. doi: 10.4048/jbc.2011.14.S.S1.
Reference
-
1.Ross JS., Fletcher JA., Linette GP., Stec J., Clark E., Ayers M, et al. The HER2-neu gene and protein in breast cancer 2003: biomarker and target of therapy. Oncologist. 2003. 8:307–25.2.Ross JS., Fletcher JA. The HER-2/neu oncogene: prognostic factor, predictive factor and target for therapy. Semin Cancer Biol. 1999. 9:125–38.3.Toikkanen S., Helin H., Isola J., Joensuu H. Prognostic significance of HER-2 oncoprotein expression in breast cancer: a 30-years follow-up. J Clin Oncol. 1992. 10:1044–8.4.Konecny G., Pauletti G., Pegram M., Untch M., Dandekar S., Aguilar Z, et al. Quantitative association between HER-2/neu and steroid hormone receptors in hormone receptor-positive primary breast cancer. J Natl Cancer Inst. 2003. 95:142–53.5.Son GM., Kwak HS., Bae YT., Sim MS., Kim JY. p53 mutation and c-erbB2 over-expression in predicting factor of responsibility to neoadjuvant chemotherapy in patients with breast cancer. J Korean Surg Soc. 2003. 65:85–94. (손경모, 곽희숙, 배영태, 심문섭, 김지연. 유방암에서술 전 화학요법의 반응 예측지표로서 c-erbB2 과발현과 p53 돌연변이의유용성. 대한외과학회지 2003;65: 85-94.).6.Nahta R., Esteva FJ. Trastuzumab: triumphs and tribulations. Oncogene. 2007. 26:3637–43.
Article7.Baselga J., Perez EA., Pienkowski T., Bell R. Adjuvant trastuzumab: a milestone in the treatment of HER-2-positive early breast cancer. Oncologist. 2006. 11:4–12.8.Piccart-Gebhart MJ., Procter M., Leyland-Jones B., Goldhirsch A., Untch M., Smith I, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005. 353:1659–72.9.Bast RC Jr., Ravdin P., Hayes DF., Bates S., Fritsche H Jr., Jessup JM, et al. 2000 update of recommendations for the use of tumor markers in breast and colorectal cancer: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol. 2001. 19:1865–78.
Article10.Wolff AC., Hammond ME., Schwartz JN., Hagerty KL., Allred DC., Cote RJ, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007. 25:118–45.
Article11.Press MF., Hung G., Godolphin W., Slamon DJ. Sensitivity of HER-2/neu antibodies in archival tissue samples: potential source of error in immunohistochemical studies of oncogene expression. Cancer Res. 1994. 54:2771–7.12.Jacobs TW., Gown AM., Yaziji H., Barnes MJ., Schnitt SJ. Specificity of HercepTest in determining HER-2/neu status of breast cancers using the United States Food and Drug Administration-approved scoring system. J Clin Oncol. 1999. 17:1983–7.13.Park K., Kim J., Lim S. Comparing fluorescence in situ hybridization and immunohistochemistry to determine the HER-2/neu status in breast carcinoma. Korean J Pathol. 2002. 36:243–8. (박경미, 김정연, 임성직. 유방암종의 HER-2/neu 검색을위한형광제자리부합화와면역조직화학염색법의비교. 대한병리학회지 2002;36: 243-8.).14.Pauletti G., Dandekar S., Rong H., Ramos L., Peng H., Seshadri R, et al. Assessment of methods for tissue-based detection of the HER-2/neu alteration in human breast cancer: a direct comparison of fluorescence in situ hybridization and immunohistochemistry. J Clin Oncol. 2000. 18:3651–64.15.Sidoni A., Ferri I., Cavaliere A., Bellezza G., Scheibel M., Bucciarelli E. Detection of HER-2/neu (c-erbB-2) overexpression and amplification in breast carcinomas with ambiguous immunohistochemical results. A further contribution to defining the role of fluorescent in situ hybridization. Anticancer Res. 2006. 26:2333–7.16.Dybdal N., Leiberman G., Anderson S., McCune B., Bajamonde A., Cohen RL, et al. Determination of HER2 gene amplification by fluorescence in situ hybridization and concordance with the clinical trials immunohistochemical assay in women with metastatic breast cancer evaluated for treatment with trastuzumab. Breast Cancer Res Treat. 2005. 93:3–11.17.Roche PC., Suman VJ., Jenkins RB., Davidson NE., Martino S., Kaufman PA, et al. Concordance between local and central laboratory HER2 testing in the breast intergroup trial N9831. J Natl Cancer Inst. 2002. 94:855–7.18.Paik S., Bryant J., Tan-Chiu E., Romond E., Hiller W., Park K, et al. Realworld performance of HER2 testing–National Surgical Adjuvant Breast and Bowel Project experience. J Natl Cancer Inst. 2002. 94:852–4.19.Perez EA., Suman VJ., Davidson NE., Martino S., Kaufman PA., Lingle WL, et al. HER2 testing by local, central, and reference laboratories in specimens from the North Central Cancer Treatment Group N9831 intergroup adjuvant trial. J Clin Oncol. 2006. 24:3032–8.20.Beatty BG., Bryant R., Wang W., Ashikaga T., Gibson PC., Leiman G, et al. HER-2/neu detection in fine-needle aspirates of breast cancer: fluorescence in situ hybridization and immunocytochemical analysis. Am J Clin Pathol. 2004. 122:246–55.21.Gu M., Ghafari S., Zhao M. Fluorescence in situ hybridization for HER-2/neu amplification of breast carcinoma in archival fine needle aspiration biopsy specimens. Acta Cytol. 2005. 19:471–6.22.Bozzetti C., Personeni N., Nizzoli R., Guazzi A., Flora M., Bassano C, et al. HER-2/neu amplification by fluorescence in situ hybridization in cytologic samples from distant metastatic sites of breast carcinoma. Cancer. 2003. 99:310–5.23.Tsukamoto F., Miyoshi Y., Koyama H., Watatani M., Sasa M., Shiba E, et al. Detection of chromosomal aneusomy by fluorescence in situ hybridization in fine-needle aspirates from breast tumors: application to the preoperative diagnosis of breast carcinoma. Cancer. 2000. 90:373–8.24.Tsuda H. HER-2 (c-erbB-2) test update: present status and problems. Breast Cancer. 2006. 13:236–48.25.Bozzetti C., Nizzoli R., Guazzi A., Flora M., Bassano C., Crafa P, et al. HER-2/neu amplification detected by fluorescence in situ hybridization in fine needle aspirates from primary breast cancer. Ann Oncol. 2002. 13:1398–403.26.Sauter G., Feichter G., Torhorst J., Moch H., Novotna H., Wagner U, et al. Fluorescence in situ hybridization for detecting erbB-2 amplification in breast tumor fine needle aspiration biopsies. Acta Cytol. 1996. 40:164–73.27.Mezzelani A., Alasio L., Bartoli C., Bonora MG., Pierotti MA., Rilke F, et al. c-erbB2/neu gene and chromosome 17 analysis in breast cancer by FISH on archival cytological fine-needle aspirates. Br J Cancer. 1999. 80:519–25.28.Nizzoli R., Bozzetti C., Crafa P., Naldi N., Guazzi A., Di Blasio B, et al. Immunocytochemical evaluation of HER-2/neu on fine-needle aspirates from primary breast carcinomas. Diagn Cytopathol. 2003. 28:142–6.29.McManus DT., Patterson AH., Maxwell P., Humphreys MW., Anderson NH. Fluorescence in situ hybridisation detection of erbB2 amplification in breast cancer fine needle aspirates. Mol Pathol. 1999. 52:75–7.30.Nizzoli R., Guazzi A., Naldi N., Fraciosi V., Bozzetti C. HER-2/neu evaluation by fluorescence in situ hybridization on destained cytologic smears from primary and metastatic breast cancer. Acta Cytol. 2005. 49:27–30.31.Latta EK., Tjan S., Parkes RK., O'Malley FP. The role of HER2/neu over-expression/amplification in the progression of ductal carcinoma in situ to invasive carcinoma of the breast. Mod Pathol. 2002. 15:1318–25.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of Silver-Enhanced in situ Hybridization and Fluorescence in situ Hybridization for HER2 Gene Status in Breast Carcinomas
- Numerical aberrations of chromosome 17 and her2/neu gene amplification, her2/neu and p 53 protein expression in breast cancer
- Prognostic Significance of HER2 Gene Amplification According to Stage of Breast Cancer
- Diagnosis and Treatment of HER2-Positive Breast Cancer
- Silver-Enhanced In Situ Hybridization as an Alternative to Fluorescence In Situ Hybridization for Assaying HER2 Amplification in Clinical Breast Cancer